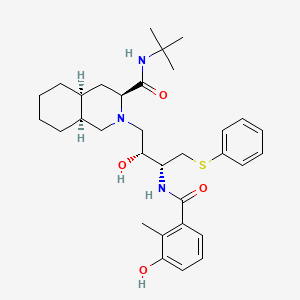
Nelfinavir
Übersicht
Beschreibung
Nelfinavir ist ein antiretrovirales Medikament, das zur Behandlung von HIV/AIDS eingesetzt wird. This compound ist ein oral bioverfügbarer Humaner-Immundefizienzvirus HIV-1-Proteaseinhibitor, der 1992 patentiert und 1997 für den medizinischen Gebrauch zugelassen wurde .
Herstellungsmethoden
This compound kann durch verschiedene Methoden synthetisiert werden. Ein Ansatz beinhaltet die Entwicklung von Nanokristallen von Nelfinavirmesilat, um die Nachteile des Arzneimittels wie schlechte Löslichkeit und orale Bioverfügbarkeit zu überwinden . Die Nanokristalle werden unter Verwendung einer kombinierten Technik und Ultraschallmethode mit Polyvinylalkohol und Poloxamer 407 als Stabilisatoren hergestellt . Die Festkörpercharakteristika der optimierten Nanokristalle werden mit Röntgenbeugung, Fourier-Transform-Infrarotspektroskopie, Differential-Scanning-Kalorimetrie und Rasterelektronenmikroskopie untersucht .
Wissenschaftliche Forschungsanwendungen
Nelfinavir hat eine breite Palette von Anwendungen in der wissenschaftlichen Forschung. Es wurde zur Behandlung von HIV-Infektionen bei Erwachsenen und Kindern eingesetzt . Darüber hinaus wurde this compound auf seine potenzielle Verwendung bei der Behandlung von COVID-19 untersucht. Klinische Studien laufen, um seine Wirksamkeit bei leichten bis mittelschweren COVID-19-Fällen zu bewerten . This compound hat sich auch als vielversprechendes Antitumormittel erwiesen, das die Zellproliferation in mehreren Krebsarten hemmt und den Zellzyklus reguliert . Darüber hinaus wurde festgestellt, dass this compound die Produktion von Virulenzfaktoren in verschiedenen Bakterien, einschließlich Streptococcus pyogenes, hemmt .
Wirkmechanismus
This compound übt seine Wirkung aus, indem es das HIV-virale Proteinaseenzym hemmt, wodurch die Spaltung des gag-pol-Polyproteins verhindert wird, was zu nicht infektiösen, unreifen viralen Partikeln führt . Die Hemmung des Protease-Enzyms ist für den HIV-Lebenszyklus unerlässlich, da sie die Zusammenlagerung unreifer Virusproteine zu reifen, infektiösen Virionen verhindert . This compound bindet an die aktive Stelle der Protease und hemmt ihre Aktivität, was zur Bildung unreifer, nicht infektiöser viraler Partikel führt .
Wirkmechanismus
Target of Action
Nelfinavir primarily targets the HIV-1 protease , an enzyme crucial for the life cycle of the Human Immunodeficiency Virus Type 1 (HIV-1) . This enzyme is responsible for the proteolytic cleavage of the viral polyprotein precursors into individual functional proteins found in infectious HIV-1 .
Mode of Action
This compound interacts with its target by binding to the protease active site, thereby inhibiting the activity of the enzyme . This inhibition prevents the cleavage of the viral polyproteins, resulting in the formation of immature, non-infectious viral particles .
Biochemical Pathways
The primary biochemical pathway affected by this compound is the HIV life cycle. By inhibiting the HIV-1 protease, this compound disrupts the proteolytic cleavage of the viral polyprotein precursors, a crucial step in the maturation of the virus . This results in the production of immature, non-infectious viral particles . Additionally, this compound has been found to induce endoplasmic reticulum stress, autophagy, and apoptosis in cancer cells .
Pharmacokinetics
This compound’s pharmacokinetic properties impact its bioavailability and efficacy. It is orally bioavailable and is metabolized in the liver by cytochrome P450 enzymes, including CYP3A4 and CYP2C19 . The elimination half-life of this compound is approximately 3.5 to 5 hours . It is primarily excreted in the feces (87%), with a small amount excreted in the urine (1-2%) . The bioavailability of this compound increases when taken with food .
Result of Action
The primary result of this compound’s action is a decrease in the amount of HIV in the blood . By inhibiting the HIV-1 protease and disrupting the maturation of the virus, this compound reduces the production of infectious HIV particles . This helps to slow the progression of HIV infection and delay the onset of AIDS .
Action Environment
The action, efficacy, and stability of this compound can be influenced by various environmental factors. For instance, the presence of food can increase the bioavailability of this compound . Additionally, the presence of other antiviral drugs can influence the effectiveness of this compound, as it is almost always used in combination with at least two other anti-HIV drugs .
Biochemische Analyse
Biochemical Properties
Nelfinavir inhibits the HIV viral proteinase enzyme which prevents cleavage of the gag-pol polyprotein, resulting in noninfectious, immature viral particles . The inhibition of the HIV-1 protease enzyme is a key interaction of this compound in biochemical reactions .
Cellular Effects
This compound’s primary cellular effect is the prevention of the formation of infectious HIV-1 particles . By inhibiting the HIV-1 protease enzyme, this compound prevents the cleavage of the gag-pol polyprotein, which is necessary for the assembly of mature, infectious HIV-1 particles .
Molecular Mechanism
This compound binds to the protease active site and inhibits the activity of the enzyme . This inhibition prevents cleavage of the viral polyproteins resulting in the formation of immature non-infectious viral particles .
Temporal Effects in Laboratory Settings
In a study analyzing the pharmacokinetic profile of this compound, it was found that there is a high variability between individuals in this compound plasma concentrations . The mean average drug plasma concentration was 2.22 ± 1.25 mg/L and the mean AUC during the dosing interval was 17.7 ± 10.0 mg•h/L .
Dosage Effects in Animal Models
While specific dosage effects in animal models are not mentioned in the available literature, it is known that this compound is used in combination with other antiviral drugs in the treatment of HIV in both adults and children .
Metabolic Pathways
This compound is metabolized by multiple cytochrome P-450 enzymes including CYP3A and CYP2C19 . One major and several minor oxidative metabolites were found in plasma .
Transport and Distribution
Unchanged this compound comprised 82-86% of the total plasma radioactivity after a single oral 750 mg dose of 14C-Nelfinavir . This suggests that this compound is primarily present in its original form in the plasma, indicating its stability during transport and distribution within the body .
Subcellular Localization
The specific subcellular localization of this compound is not mentioned in the available literature. Given its role as a protease inhibitor, it is likely that this compound interacts with the HIV-1 protease enzyme in the cytoplasm of the cell, where the assembly of new viral particles takes place .
Vorbereitungsmethoden
Nelfinavir can be synthesized through various methods. One approach involves the development of nanocrystals of this compound Mesylate to overcome the drawbacks associated with the drug, such as poor solubility and oral bioavailability . The nanocrystals are prepared using a combination technique and ultrasonication method with polyvinyl alcohol and poloxamer 407 as stabilizers . The solid-state characteristics of the optimized nanocrystals are studied using X-ray diffraction, Fourier-transform infrared spectroscopy, differential scanning calorimetry, and scanning electron microscopy .
Analyse Chemischer Reaktionen
Nelfinavir durchläuft verschiedene chemische Reaktionen, darunter Oxidation, Reduktion und Substitution. Es ist ein Proteaseinhibitor mit Aktivität gegen das humane Immundefizienzvirus Typ 1 (HIV-1) . Proteaseinhibitoren blockieren den Teil von HIV, der als Protease bezeichnet wird, ein Enzym, das für die proteolytische Spaltung der viralen Polyproteinvorläufer in die einzelnen funktionellen Proteine benötigt wird, die in infektiösem HIV-1 gefunden werden . This compound bindet an die aktive Stelle der Protease und hemmt die Aktivität des Enzyms, wodurch die Spaltung der viralen Polyproteine verhindert und die Bildung unreifer, nicht infektiöser viraler Partikel bewirkt wird .
Vergleich Mit ähnlichen Verbindungen
Nelfinavir ist unter den Proteaseinhibitoren aufgrund seiner spezifischen Struktur und seines Wirkmechanismus einzigartig. Ähnliche Verbindungen umfassen andere Proteaseinhibitoren wie Ritonavir, Lopinavir und Indinavir . Diese Verbindungen hemmen auch das HIV-Protease-Enzym, können sich jedoch in ihren pharmakokinetischen Eigenschaften, Nebenwirkungen und Wirksamkeit unterscheiden . This compound hat sich als eine einzigartige cis-Decahydroisoquinolin-2-carboxamid-Einheit erwiesen, die die strukturelle Grundlage für seine erhöhte Wirksamkeit gegen Krebs im Vergleich zu anderen HIV-Proteaseinhibitoren liefern könnte .
Eigenschaften
IUPAC Name |
(3S,4aS,8aS)-N-tert-butyl-2-[(2R,3R)-2-hydroxy-3-[(3-hydroxy-2-methylbenzoyl)amino]-4-phenylsulfanylbutyl]-3,4,4a,5,6,7,8,8a-octahydro-1H-isoquinoline-3-carboxamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C32H45N3O4S/c1-21-25(15-10-16-28(21)36)30(38)33-26(20-40-24-13-6-5-7-14-24)29(37)19-35-18-23-12-9-8-11-22(23)17-27(35)31(39)34-32(2,3)4/h5-7,10,13-16,22-23,26-27,29,36-37H,8-9,11-12,17-20H2,1-4H3,(H,33,38)(H,34,39)/t22-,23+,26-,27-,29+/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
QAGYKUNXZHXKMR-HKWSIXNMSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1=C(C=CC=C1O)C(=O)NC(CSC2=CC=CC=C2)C(CN3CC4CCCCC4CC3C(=O)NC(C)(C)C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC1=C(C=CC=C1O)C(=O)N[C@@H](CSC2=CC=CC=C2)[C@@H](CN3C[C@H]4CCCC[C@H]4C[C@H]3C(=O)NC(C)(C)C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C32H45N3O4S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
159989-65-8 (monomethane sulfonate (salt)) | |
| Record name | Nelfinavir [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0159989647 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID5035080 | |
| Record name | Nelfinavir | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5035080 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
567.8 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Nelfinavir | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014365 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Slightly soluble, 1.91e-03 g/L | |
| Record name | Nelfinavir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00220 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Nelfinavir | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014365 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
HIV viral protease is an important enzyme for HIV maturation and pathogenicity since HIV produces its structural and key proteins in the form of a polyprotein that needs to be cleaved by a protease. HIV protease is synthesized as part of the Gag-pol polyprotein, where Gag encodes for the capsid and matrix protein to form the outer protein shell, and Pol encodes for the reverse transcriptase and integrase protein to synthesize and incorporate its genome into host cells. The Gag-pol polyprotein undergoes proteolytic cleavage by HIV protease to produce 66 molecular species which will assume conformational changes to become fully active. Inhibition of protease, therefore, prevents HIV virion from fully maturing and becoming infective. Nelfinavir is a competitive inhibitor of the HIV protease by reversibly binding to the active site of the enzyme, preventing it from interacting with its substrate to produce mature and infectious viral particles. | |
| Record name | Nelfinavir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00220 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
159989-64-7 | |
| Record name | Nelfinavir | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=159989-64-7 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Nelfinavir [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0159989647 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Nelfinavir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00220 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | nelfinavir | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=747167 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Nelfinavir | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5035080 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | NELFINAVIR | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/HO3OGH5D7I | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Nelfinavir | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014365 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
349.84 °C | |
| Record name | Nelfinavir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00220 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Nelfinavir | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014365 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary mechanism of action of Nelfinavir?
A1: this compound was initially developed as a specific inhibitor of human immunodeficiency virus (HIV) protease. [] It exerts its antiviral activity by binding to the active site of the HIV-1 protease, thereby preventing the cleavage of viral polyproteins and inhibiting viral maturation. []
Q2: How does this compound's activity extend to anti-cancer effects?
A2: Beyond its antiviral activity, this compound exhibits pleiotropic effects in various cancer cells. [, ] One key mechanism involves the induction of endoplasmic reticulum (ER) stress, leading to the unfolded protein response (UPR) and ultimately apoptosis (programmed cell death). [, , ] Additionally, this compound inhibits the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway, a key regulator of cell growth and survival, further contributing to its anti-cancer properties. [, ]
Q3: this compound has been shown to affect lipid metabolism in cancer cells. Can you elaborate?
A3: this compound inhibits the activity of Site-2 protease (S2P), an enzyme involved in the regulated intramembrane proteolysis (RIP) of sterol regulatory element binding protein-1 (SREBP-1) and activating transcription factor 6 (ATF6). [] This inhibition disrupts lipid homeostasis, contributing to ER stress and promoting cell death.
Q4: Can you explain the role of this compound in modulating the unfolded protein response (UPR) and its impact on cancer cells?
A4: this compound activates the UPR in myeloma cells, leading to the upregulation of UPR-related proteins. [] This activation can overcome proteasome inhibitor resistance often observed in multiple myeloma. [] The exact mechanisms underlying this effect are complex but involve modulation of key UPR sensors and downstream effectors.
Q5: How does this compound impact the tumor microenvironment?
A5: this compound downregulates hypoxia-inducible factor 1-alpha (HIF-1α) and vascular endothelial growth factor (VEGF) expression, leading to decreased angiogenesis (formation of new blood vessels). [] This, in turn, increases tumor oxygenation, potentially enhancing the efficacy of radiotherapy. []
Q6: What is the role of oxidative stress in this compound's anticancer activity?
A6: this compound has been shown to increase reactive oxygen species (ROS) production in breast cancer cells, leading to disruption of the Akt/HSP90 complex and subsequent Akt degradation, ultimately contributing to cell death. [] This ROS-dependent mechanism appears to be selective for cancer cells, sparing normal breast cells. []
Q7: What is the molecular formula and weight of this compound?
A7: this compound mesylate, the salt form commonly used in formulations, has the molecular formula C32H45N3O4S • CH4O3S and a molecular weight of 663.87 g/mol.
Q8: Have any computational studies been performed on this compound?
A8: Yes, computational methods have been used to develop a pharmacophore model for this compound interaction with the multidrug resistance protein 4 (MRP4/ABCC4). [] This model revealed that this compound shares a binding site with chemotherapeutic agents like adefovir and methotrexate, highlighting its potential as both a substrate and inhibitor of this transporter. []
Q9: What is the typical route of administration and absorption profile of this compound?
A10: this compound is administered orally. [, , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , ] Food significantly affects its absorption, with a high-calorie meal required to achieve optimal plasma concentrations. []
Q10: How is this compound metabolized in the body?
A11: this compound undergoes extensive metabolism in the liver, primarily by cytochrome P450 (CYP) enzymes, mainly CYP3A4 and CYP2C19. [, ] The major metabolite, this compound hydroxy-t-butylamide (M8), exhibits potent antiviral activity. [, , ]
Q11: What factors can influence the pharmacokinetics of this compound?
A11: Various factors can influence this compound pharmacokinetics, including age, co-administered medications, and liver function. For instance:
- Age: Children, especially infants, exhibit different pharmacokinetic profiles compared to adults, generally requiring higher doses to achieve similar drug exposure. [, , ]
- Co-administered drugs: Co-administration with other protease inhibitors can lead to significant drug interactions, altering the plasma concentrations of both drugs. [, , ] Similarly, drugs that induce or inhibit CYP3A4 can impact this compound metabolism. []
- Liver disease: Liver disease, especially moderate to severe, can impair this compound metabolism, leading to lower M8 formation and requiring dose adjustments. []
Q12: How do this compound plasma concentrations relate to its efficacy in treating HIV?
A13: Studies suggest a correlation between this compound plasma concentrations and virological treatment success in HIV-infected individuals. Patients with low this compound levels are at an increased risk of virologic failure. [] This finding highlights the importance of therapeutic drug monitoring to optimize treatment outcomes.
Q13: What types of in vitro models have been used to study this compound?
A14: Various in vitro models, including cell lines derived from different cancer types (e.g., breast, lung, ovarian, multiple myeloma) have been used to investigate this compound's anti-cancer effects. [, , , , , , ] These studies have provided insights into its mechanisms of action, such as induction of apoptosis, ER stress, and inhibition of specific signaling pathways. [, , , , , ]
Q14: What in vivo models have been employed to evaluate this compound's anti-cancer potential?
A15: this compound's in vivo efficacy has been assessed in various animal models, including mouse xenograft models of different cancer types. [, , , ] These studies have demonstrated that this compound can inhibit tumor growth in vivo, often in conjunction with markers of ER stress, autophagy, and apoptosis. [, , , ]
Q15: Have any clinical trials been conducted with this compound in cancer patients?
A15: Yes, several clinical trials have explored this compound's potential as an anti-cancer agent in humans. For instance:
- Phase I trials: These trials have primarily focused on establishing the maximum tolerated dose (MTD) and safety profile of this compound in patients with advanced solid tumors. [] Results indicate that this compound is generally well-tolerated at doses higher than those used for HIV treatment. []
- Phase I/II trials: A trial combining this compound with bortezomib in patients with advanced hematologic malignancies demonstrated promising activity, particularly in bortezomib-refractory multiple myeloma. []
Q16: What are the primary HIV protease mutations associated with this compound resistance?
A17: The D30N mutation is the most common primary resistance mutation selected for by this compound. [, ] While it confers minimal cross-resistance to other protease inhibitors, the emergence of L90M, often accompanied by secondary mutations, can lead to broad cross-resistance within the protease inhibitor class. []
Q17: How does this compound resistance impact subsequent treatment options for HIV?
A18: Interestingly, despite the emergence of D30N, many patients experiencing virologic failure on a this compound-containing regimen can successfully switch to another protease inhibitor and achieve viral suppression. [, ] This observation suggests that the development of cross-resistance, while possible, is not inevitable.
Q18: Is there any evidence of cross-resistance between this compound and other anti-cancer agents?
A19: While this compound demonstrates synergism with some chemotherapeutic agents, like bortezomib, [, ] specific information regarding cross-resistance patterns with other anti-cancer drugs is limited within the provided research papers.
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.


![[4-chloro-3-[5-methyl-3-[4-(2-pyrrolidin-1-ylethoxy)anilino]-1,2,4-benzotriazin-7-yl]phenyl] benzoate;hydrochloride](/img/structure/B1663545.png)
![2-[5-[3-[4-(2-bromo-5-fluorophenoxy)piperidin-1-yl]-1,2-oxazol-5-yl]tetrazol-2-yl]acetic acid](/img/structure/B1663549.png)
![3-[4-[[4-(2-chlorophenyl)-1,3-thiazol-2-yl]methoxy]-2-methylphenyl]propanoic acid](/img/structure/B1663568.png)
