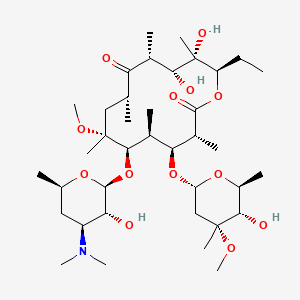
Clarithromycin
Übersicht
Beschreibung
Clarithromycin ist ein Makrolid-Antibiotikum, das von Erythromycin abgeleitet ist. Es wird häufig zur Behandlung verschiedener bakterieller Infektionen eingesetzt, darunter Streptokokken-Angina, Lungenentzündung, Hautinfektionen, Helicobacter pylori-Infektion und Lyme-Borreliose . This compound wirkt, indem es die bakterielle Proteinbiosynthese hemmt, wodurch es eine effektive Behandlung für eine breite Palette bakterieller Infektionen darstellt .
Herstellungsmethoden
This compound wird aus Erythromycin durch eine Reihe chemischer Reaktionen synthetisiert. Der Prozess umfasst Oximierung, Veretherung, Silanisierung, Methylierung und Reduktionshydrolyse . Die industrielle Produktion von this compound umfasst in der Regel die folgenden Schritte:
Oximierungsreaktion: Erythromycinthiocyanat unterliegt einer Oximierungsreaktion.
Veretherungsreaktion: Das Oxim wird dann verethert.
Silanierungsreaktion: Das veretherte Produkt wird einer Silanisierung unterzogen.
Methylierungsreaktion: Das silanierte Produkt wird einer Methylierung unterzogen.
Reduktionshydrolyse: Schließlich wird das Produkt reduziert und hydrolysiert, um this compound zu erhalten.
Chemische Reaktionsanalyse
This compound unterliegt verschiedenen chemischen Reaktionen, darunter:
Oxidation: this compound kann unter bestimmten Bedingungen oxidiert werden.
Reduktion: Reduktionsreaktionen sind Teil seines Syntheseprozesses.
Substitution: Substitutionsreaktionen können auftreten, insbesondere während seiner Synthese.
Häufige Reagenzien, die in diesen Reaktionen verwendet werden, sind organische Lösungsmittel, Säuren und Basen. Die Hauptprodukte, die aus diesen Reaktionen gebildet werden, sind Zwischenprodukte, die zum Endprodukt this compound führen .
Wirkmechanismus
Target of Action
Clarithromycin is a macrolide antibiotic . Its primary target is the bacterial 50S ribosomal subunit . This subunit is a crucial component of the bacterial protein synthesis machinery, and its inhibition disrupts the production of essential proteins that bacteria need for survival and replication .
Mode of Action
This compound inhibits bacterial protein synthesis by binding to the bacterial 50S ribosomal subunit . This binding inhibits peptidyl transferase activity and interferes with amino acid translocation during the translation and protein assembly process . Depending on the organism and drug concentration, this compound may be bacteriostatic (inhibits bacterial growth) or bactericidal (kills bacteria) .
Biochemical Pathways
This compound’s action primarily affects the protein synthesis pathway in bacteria . By binding to the 50S ribosomal subunit, it disrupts the translation process, leading to a decrease in bacterial growth . Additionally, research suggests that this compound may alter glycerophospholipid metabolism in certain contexts .
Pharmacokinetics
This compound is well absorbed from the gastrointestinal tract, and its systemic bioavailability is about 55%, which is reduced due to first-pass metabolism . It undergoes rapid biodegradation to produce the microbiologically active 14-hydroxy-®-metabolite . The maximum serum concentrations of this compound and its 14-hydroxy metabolite appear within 2-3 hours . This compound is well distributed throughout the body and achieves higher concentrations in tissues than in the blood . The main metabolic pathways are oxidative N-demethylation and hydroxylation, which are saturable and result in nonlinear pharmacokinetics .
Result of Action
The molecular and cellular effects of this compound’s action include the inhibition of bacterial protein synthesis, leading to a decrease in bacterial growth . This disruption of protein synthesis can lead to bacterial cell death, thereby helping the body’s immune system eliminate the infection .
Action Environment
Environmental factors can influence the action, efficacy, and stability of this compound. For instance, the presence of other antimicrobials in the environment can exacerbate antimicrobial contamination and the spread of antimicrobial resistance . Furthermore, this compound’s effectiveness can be influenced by the pH of the environment, as it is more stable under acidic conditions .
Wissenschaftliche Forschungsanwendungen
Clarithromycin hat zahlreiche Anwendungen in der wissenschaftlichen Forschung:
Chemie: Wird als Modellverbindung zur Untersuchung von Makrolid-Antibiotika verwendet.
Biologie: Untersucht hinsichtlich seiner Auswirkungen auf die bakterielle Proteinbiosynthese.
Industrie: Wird in der pharmazeutischen Industrie zur Herstellung von Antibiotika verwendet.
Wirkmechanismus
This compound entfaltet seine Wirkung, indem es an die bakterielle 50S-ribosomale Untereinheit bindet. Diese Bindung hemmt die Peptidyltransferaseaktivität und stört den Aminosäuretransport während des Translationsprozesses und der Proteinassemblierung. Dadurch wird die bakterielle Proteinbiosynthese gehemmt, was zu einer Abnahme des bakteriellen Wachstums führt und letztendlich dem Immunsystem des Körpers hilft, die Infektion zu eliminieren .
Biochemische Analyse
Biochemical Properties
Clarithromycin is well absorbed from the gastrointestinal tract and its systemic bioavailability (about 55%) is reduced because of first-pass metabolism . It undergoes rapid biodegradation to produce the microbiologically active 14-hydroxy-®-metabolite .
Cellular Effects
This compound is indicated for the treatment of bacterial infection associated with sinusitis, tonsillitis, pneumonia, acne (vulgari), and the advanced stage of HIV infections in AIDS patients . In combination with amoxicillin and a proton inhibitor drug, it is used effectively in duodenal ulcer treatment to eradicate helicobacter pylori in a short period of time .
Molecular Mechanism
The main metabolic pathways of this compound are oxidative N-demethylation and hydroxylation, which are saturable and result in nonlinear pharmacokinetics . The primary metabolite (14-hydroxy derivative) is mainly excreted in the urine with the parent compound .
Temporal Effects in Laboratory Settings
A selective, sensitive, and stability-indicating reversed-phase high-performance liquid chromatography method was developed and validated for the determination of this compound antibiotic in human plasma . Stock solutions and calibration standards of the drug and quality control preparations were demonstrated to be stable at room temperature and –20°C for long and short periods of time .
Metabolic Pathways
This compound is involved in metabolic pathways that include oxidative N-demethylation and hydroxylation . The primary metabolite (14-hydroxy derivative) is mainly excreted in the urine with the parent compound .
Transport and Distribution
This compound is well distributed throughout the body and achieves higher concentrations in tissues than in the blood . Also, the 14-hydroxy metabolite exhibits high tissue concentrations, with values about one-third of the parent compound concentrations .
Vorbereitungsmethoden
Clarithromycin is synthesized from erythromycin through a series of chemical reactions. The process involves oximation, etherification, silanization, methylation, and reduction hydrolysis . The industrial production of this compound typically involves the following steps:
Oximation Reaction: Erythromycin thiocyanate undergoes an oximation reaction.
Etherification Reaction: The oxime is then etherified.
Silanization Reaction: The etherified product is subjected to silanization.
Methylation Reaction: The silanized product undergoes methylation.
Reduction Hydrolysis: Finally, the product is reduced and hydrolyzed to obtain this compound.
Analyse Chemischer Reaktionen
Clarithromycin undergoes various chemical reactions, including:
Oxidation: this compound can be oxidized under specific conditions.
Reduction: Reduction reactions are part of its synthesis process.
Substitution: Substitution reactions can occur, particularly during its synthesis.
Common reagents used in these reactions include organic solvents, acids, and bases. The major products formed from these reactions are intermediates that lead to the final product, this compound .
Vergleich Mit ähnlichen Verbindungen
Clarithromycin ähnelt anderen Makrolid-Antibiotika wie Erythromycin und Azithromycin. Es hat jedoch einzigartige Eigenschaften, die es von diesen unterscheiden:
Erythromycin: This compound ist ein 6-O-Methyl-Derivat von Erythromycin, das seine Stabilität und Bioverfügbarkeit erhöht.
Azithromycin: Obwohl beide Makrolide sind, hat this compound ein breiteres Wirkungsspektrum und ist gegen bestimmte Bakterienstämme effektiver.
Zu ähnlichen Verbindungen gehören:
- Erythromycin
- Azithromycin
- Amoxicillin
- Augmentin (Amoxicillin/Clavulansäure)
Die einzigartige chemische Struktur und die verbesserte Stabilität von this compound machen es zu einem wertvollen Antibiotikum bei der Behandlung verschiedener bakterieller Infektionen.
Eigenschaften
IUPAC Name |
(3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-6-[(2S,3R,4S,6R)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-14-ethyl-12,13-dihydroxy-4-[(2R,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyloxan-2-yl]oxy-7-methoxy-3,5,7,9,11,13-hexamethyl-oxacyclotetradecane-2,10-dione | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C38H69NO13/c1-15-26-38(10,45)31(42)21(4)28(40)19(2)17-37(9,47-14)33(52-35-29(41)25(39(11)12)16-20(3)48-35)22(5)30(23(6)34(44)50-26)51-27-18-36(8,46-13)32(43)24(7)49-27/h19-27,29-33,35,41-43,45H,15-18H2,1-14H3/t19-,20-,21+,22+,23-,24+,25+,26-,27+,29-,30+,31-,32+,33-,35+,36-,37-,38-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
AGOYDEPGAOXOCK-KCBOHYOISA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCC1C(C(C(C(=O)C(CC(C(C(C(C(C(=O)O1)C)OC2CC(C(C(O2)C)O)(C)OC)C)OC3C(C(CC(O3)C)N(C)C)O)(C)OC)C)C)O)(C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC[C@@H]1[C@@]([C@@H]([C@H](C(=O)[C@@H](C[C@@]([C@@H]([C@H]([C@@H]([C@H](C(=O)O1)C)O[C@H]2C[C@@]([C@H]([C@@H](O2)C)O)(C)OC)C)O[C@H]3[C@@H]([C@H](C[C@H](O3)C)N(C)C)O)(C)OC)C)C)O)(C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C38H69NO13 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID3022829 | |
| Record name | Clarithromycin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3022829 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
748.0 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Clarithromycin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015342 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
2.17e-01 g/L | |
| Record name | Clarithromycin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01211 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Clarithromycin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015342 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Clarithromycin is first metabolized to 14-OH clarithromycin, which is active and works synergistically with its parent compound. Like other macrolides, it then penetrates bacteria cell wall and reversibly binds to domain V of the 23S ribosomal RNA of the 50S subunit of the bacterial ribosome, blocking translocation of aminoacyl transfer-RNA and polypeptide synthesis. Clarithromycin also inhibits the hepatic microsomal CYP3A4 isoenzyme and P-glycoprotein, an energy-dependent drug efflux pump., Clarithromycin usually is bacteriostatic, although it may be bactericidal in high concentrations or against highly susceptible organisms. Bactericidal activity has been observed against Streptococcus pyogenes, S. pneumoniae, Haemophilus influenzae, and Chlamydia trachomatis. Clarithromycin inhibits protein synthesis in susceptible organisms by penetrating the cell wall and binding to 50S ribosomal subunits, thereby inhibiting translocation of aminoacyl transfer-RNA and inhibiting polypeptide synthesis. The site of action of clarithromycin appears to be the same as that of erythromycin, clindamycin, lincomycin, and chloramphenicol. | |
| Record name | Clarithromycin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01211 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Clarithromycin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8055 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Colorless needles from chloroform + diisopropyl ether (1:2) ... Also reported as crystals from ethanol | |
CAS No. |
81103-11-9, 116836-41-0 | |
| Record name | Clarithromycin | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=81103-11-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Clarithromycin [USAN:USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0081103119 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | (14R)-14-Hydroxyclarithromycin | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0116836410 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Clarithromycin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01211 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | clarithromycin | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=758704 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Clarithromycin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3022829 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Erythromycin, 6-O-methyl | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.119.644 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | Clarithromycin | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | CLARITHROMYCIN | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/H1250JIK0A | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Clarithromycin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8055 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Clarithromycin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015342 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
217-220 °C (decomposes) ... Also reported as mp 222-225 °C, 217 - 220 °C | |
| Record name | Clarithromycin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01211 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Clarithromycin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8055 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Clarithromycin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015342 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary mechanism of action of clarithromycin?
A1: this compound exerts its antibacterial effect by binding to the 23S ribosomal RNA (rRNA) of susceptible bacteria. [, , , ] This binding interferes with bacterial protein synthesis, ultimately leading to bacterial growth inhibition or death. [, ]
Q2: Why is this compound effective against a wide range of bacterial species?
A2: this compound exhibits activity against a broad spectrum of bacteria, encompassing both Gram-positive and some Gram-negative species. [] This is largely attributed to the conserved nature of the 23S rRNA target across different bacterial species. []
Q3: Does this compound exhibit bactericidal or bacteriostatic activity?
A3: this compound's activity can be both bactericidal (killing bacteria) and bacteriostatic (inhibiting bacterial growth) depending on the bacterial species, the concentration of the antibiotic, and the site of infection. [, ] For example, this compound demonstrates bactericidal activity against Haemophilus influenzae and some strains of Mycobacterium avium. [, ]
Q4: How does the activity of this compound's primary metabolite, 14-hydroxythis compound, compare to the parent drug?
A4: 14-hydroxythis compound, the major metabolite of this compound in humans, also possesses antibacterial activity. [, ] Interestingly, this metabolite often exhibits greater potency against certain bacteria, such as Haemophilus influenzae, compared to this compound itself. [, ]
Q5: What is the molecular formula and weight of this compound?
A5: this compound is represented by the molecular formula C38H69NO13. It has a molecular weight of 747.95 g/mol. [, ]
Q6: How stable is this compound in acidic environments?
A6: this compound demonstrates instability in low pH solutions. [] Research indicates that its degradation rate increases with decreasing pH. []
Q7: Can the stability of this compound in acidic conditions be improved?
A7: Yes, incorporating polymers like Carbopol 934p and ethylcellulose into this compound formulations provides a protective effect against degradation in low pH environments. [] This suggests that formulation strategies can significantly impact the stability of this compound.
Q8: What is the primary mechanism of resistance to this compound in bacteria like Helicobacter pylori?
A8: The most prevalent mechanism of this compound resistance, particularly in Helicobacter pylori, involves point mutations within the 23S rRNA gene. [, , , , , ] These mutations, often occurring at positions A2142G, A2143G, and A2144G, alter the binding site of this compound, reducing its efficacy. [, , , , ]
Q9: Is there a correlation between the specific 23S rRNA mutation and the level of this compound resistance?
A9: Yes, different point mutations within the 23S rRNA gene can lead to varying degrees of resistance to this compound. [, ] For instance, the A2143G mutation is frequently associated with higher levels of resistance compared to the A2142G mutation. [, ]
Q10: Does this compound resistance impact the effectiveness of combination therapies for Helicobacter pylori eradication?
A10: Yes, the presence of this compound-resistant Helicobacter pylori strains significantly diminishes the success rate of this compound-containing eradication therapies. [, , , , , , ] This highlights the importance of considering antibiotic resistance profiles when selecting treatment regimens.
Q11: How is this compound metabolized in the body?
A11: this compound undergoes significant first-pass metabolism in the liver, primarily by cytochrome P450 enzymes, leading to the formation of several metabolites, including the microbiologically active 14-hydroxythis compound. [, , ]
Q12: How does the pharmacokinetic profile of this compound change with repeated dosing?
A12: this compound exhibits non-linear pharmacokinetics, meaning that its elimination half-life and area under the curve (AUC) do not increase proportionally with increasing doses. [] This is thought to be partly due to the saturation of metabolic enzymes involved in its metabolism. []
Q13: Does this compound interact with other drugs that are metabolized by cytochrome P450 enzymes?
A13: Yes, this compound can inhibit the activity of cytochrome P450 3A4, a major drug-metabolizing enzyme. [, ] This inhibition can elevate the plasma concentrations of other drugs metabolized by this enzyme, potentially leading to increased risk of adverse effects. [, ]
Q14: How does this compound's efficacy against MAC infections compare to other antibiotics?
A14: this compound has shown promise in treating MAC infections, but its efficacy compared to other antibiotics, such as rifampicin and ethambutol, varies depending on the specific MAC species, the severity of infection, and the patient population. [, , , ]
Q15: What are the potential benefits of using liposomal formulations of this compound?
A15: Liposomal formulations of this compound have been explored as a way to enhance its efficacy, particularly against resistant strains of Pseudomonas aeruginosa. [] Encapsulating this compound within liposomes can improve its delivery to target cells and reduce its toxicity. []
Q16: What analytical techniques are commonly employed to determine this compound concentrations in biological samples?
A16: High-performance liquid chromatography (HPLC) coupled with various detection methods, such as UV detection or tandem mass spectrometry (LC-MS/MS), are frequently used to quantify this compound and its metabolites in biological matrices like plasma and serum. [, , , ]
Q17: How is this compound resistance typically assessed in laboratory settings?
A17: this compound resistance can be assessed through phenotypic methods like the Etest or agar dilution, which measure the minimum inhibitory concentration (MIC) of the antibiotic required to inhibit bacterial growth. [, , , ] Genotypic methods, such as polymerase chain reaction (PCR)-based techniques, are also employed to detect specific mutations in the 23S rRNA gene associated with this compound resistance. [, , , ]
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.