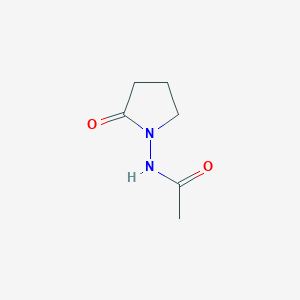
Piracetam
Übersicht
Beschreibung
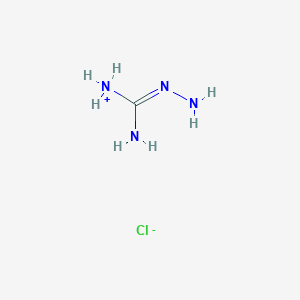
Piracetam ist ein Nootropikum, das zur Gruppe der Racetame gehört und die chemische Bezeichnung 2-Oxo-1-Pyrrolidin-acetamid trägt. Es ist ein zyklisches Derivat des Neurotransmitters Gamma-Aminobuttersäure (GABA). This compound wurde erstmals 1971 von UCB Pharma vermarktet und ist bekannt für seine kognitionsfördernden Eigenschaften. Es wird bei verschiedenen kognitiven Störungen, Schwindel, kortikaler Myoklonus, Legasthenie und Sichelzellanämie eingesetzt .
Herstellungsmethoden
Synthesewege und Reaktionsbedingungen
This compound kann durch verschiedene Verfahren synthetisiert werden. Eine gängige Methode beinhaltet die Reaktion von 2-Pyrrolidon mit Ethylchloracetat in Gegenwart einer Base, gefolgt von der Hydrolyse zu this compound. Ein weiteres Verfahren beinhaltet die Cyclisierung von Gamma-Aminobuttersäure (GABA) mit Essigsäureanhydrid .
Industrielle Produktionsmethoden
Die industrielle Produktion von this compound erfolgt üblicherweise in großem Maßstab unter Verwendung der oben genannten Verfahren. Der Prozess wird auf hohe Ausbeute und Reinheit optimiert, um sicherzustellen, dass das Endprodukt pharmazeutische Standards erfüllt. Der Produktionsprozess umfasst Schritte wie Kristallisation, Filtration und Trocknung, um die reine Verbindung zu erhalten .
Wirkmechanismus
Target of Action
Piracetam is a cyclic derivative of the neurotransmitter γ-aminobutyric acid (GABA) and shares the same 2-oxo-pyrrolidone base structure with pyroglutamic acid . Its mechanism of action differs from that of endogenous gaba .
Mode of Action
This compound’s mode of action involves modulation of neurotransmitters, enhanced brain metabolism, improved blood flow, neuroprotection, and facilitation of neuronal communication . It also has effects on the vascular system by reducing erythrocyte adhesion to the vascular endothelium, hindering vasospasms, and facilitating microcirculation .
Biochemical Pathways
This compound has been found to inhibit the PI3K/Akt/mTOR pathway in OGD-stimulated SH-SY5Y cells . This pathway is involved in cell cycle regulation and is often dysregulated in diseases
Wissenschaftliche Forschungsanwendungen
Piracetam hat eine breite Palette von Anwendungen in der wissenschaftlichen Forschung:
Chemie: Als Modellverbindung zur Untersuchung der Eigenschaften von cyclischen Amiden und ihren Derivaten.
Biologie: Untersucht wegen seiner Auswirkungen auf die Fluidität der Zellmembran und den Neuro-Schutz.
Medizin: Wird zur Behandlung von kognitiven Störungen, Myoklonus und Sichelzellanämie eingesetzt. Es wurde auch wegen seiner potenziellen Vorteile bei Erkrankungen wie Demenz, Schwindel und Legasthenie untersucht.
Industrie: Wird bei der Entwicklung von Nootropika und kognitionsfördernden Mitteln eingesetzt .
Wirkmechanismus
Der Wirkmechanismus von this compound ist nicht vollständig geklärt, es wird jedoch vermutet, dass er mehrere Wege einschließt:
Neurotransmittermodulation: this compound moduliert die Neurotransmission in cholinergen und glutamatergen Systemen.
Neuro-Schutz: Es hat neuroprotektive Eigenschaften, die die neuronale Plastizität verbessern und vor Hypoxie schützen.
Vaskuläre Wirkungen: This compound reduziert die Erythrozytenadhäsion am vaskulären Endothel, hemmt Vasospasmen und fördert die Mikrozirkulation .
Biochemische Analyse
Biochemical Properties
Piracetam interacts with various enzymes, proteins, and other biomolecules. It shares the same 2-oxo-pyrrolidone base structure with pyroglutamic acid and is a cyclic derivative of the neurotransmitter γ-aminobutyric acid (GABA) . Its mechanism of action differs from that of endogenous GABA . This compound has neuroprotective and anticonvulsant properties and is reported to improve neural plasticity .
Cellular Effects
This compound has significant effects on various types of cells and cellular processes. It enhances the growth of cells, inhibits oxidative stress, and improves mitochondrial function . It influences cell function by modulating neurotransmission in a range of transmitter systems, including cholinergic and glutamatergic systems .
Molecular Mechanism
This compound exerts its effects at the molecular level through various mechanisms. It influences neuronal and vascular functions by restoring cell membrane fluidity . This mechanism of action is thought to improve membrane stability, allowing the membrane and transmembrane proteins to maintain and recover the three-dimensional structure or folding for normal function .
Temporal Effects in Laboratory Settings
This compound shows changes in its effects over time in laboratory settings. It has been observed that this compound increases regional cerebral blood flow . This suggests that this compound might have long-term effects on cellular function observed in in vitro or in vivo studies .
Dosage Effects in Animal Models
The effects of this compound vary with different dosages in animal models. For instance, daily this compound treatment at doses of 0, 75, 150, and 300 mg/kg ip was initiated in 6-week-old male mice. The study found that low doses of this compound reduced search time in the visible-platform component, while all this compound doses prevented trial-related improvements in performance .
Metabolic Pathways
This compound is involved in various metabolic pathways. It has been shown to alter the physical properties of the plasma membrane by increasing its fluidity and protecting the cell against hypoxia . It also increases red cell deformability and normalizes the aggregation of hyperactive platelets .
Transport and Distribution
This compound is transported and distributed within cells and tissues. After oral ingestion, this compound is rapidly absorbed with a bioavailability of 100%. Its volume of distribution is 0.6 L/kg and plasma protein binding is 0% .
Subcellular Localization
It is known that this compound influences neuronal and vascular functions by restoring cell membrane fluidity . This suggests that this compound might have specific targeting signals or post-translational modifications that direct it to specific compartments or organelles.
Vorbereitungsmethoden
Synthetic Routes and Reaction Conditions
Piracetam can be synthesized through several methods. One common method involves the reaction of 2-pyrrolidone with ethyl chloroacetate in the presence of a base, followed by hydrolysis to yield this compound. Another method involves the cyclization of gamma-aminobutyric acid (GABA) with acetic anhydride .
Industrial Production Methods
Industrial production of this compound typically involves large-scale synthesis using the aforementioned methods. The process is optimized for high yield and purity, ensuring that the final product meets pharmaceutical standards. The production process includes steps such as crystallization, filtration, and drying to obtain the pure compound .
Analyse Chemischer Reaktionen
Arten von Reaktionen
Piracetam unterliegt verschiedenen chemischen Reaktionen, darunter:
Oxidation: this compound kann zu 2-Pyrrolidon-5-carbonsäure oxidiert werden.
Reduktion: Die Reduktion von this compound kann 2-Pyrrolidon ergeben.
Substitution: Substitutionsreaktionen können an der Acetamidgruppe auftreten und zur Bildung verschiedener Derivate führen
Häufige Reagenzien und Bedingungen
Oxidation: Häufige Oxidationsmittel sind Kaliumpermanganat und Wasserstoffperoxid.
Reduktion: Reduktionsmittel wie Lithiumaluminiumhydrid können verwendet werden.
Substitution: Reagenzien wie Alkylhalogenide und Acylchloride werden üblicherweise für Substitutionsreaktionen verwendet
Hauptprodukte, die gebildet werden
Oxidation: 2-Pyrrolidon-5-carbonsäure
Reduktion: 2-Pyrrolidon
Substitution: Verschiedene Acetamidderivate
Vergleich Mit ähnlichen Verbindungen
Piracetam wird oft mit anderen Racetamen verglichen, darunter:
Oxiracetam: Bekannt für seine stimulierenden Wirkungen und zur kognitiven Verbesserung eingesetzt.
Aniracetam: Hat anxiolytische Eigenschaften und wird bei Angstzuständen und kognitiven Störungen eingesetzt.
Pramiracetam: Potenter als this compound, zur Behandlung von kognitiven Defiziten im Zusammenhang mit traumatischen Hirnverletzungen eingesetzt.
Phenylthis compound: Potenter und für eine größere Bandbreite an Indikationen eingesetzt, darunter die Steigerung der kognitiven Leistung und der körperlichen Leistungsfähigkeit .
This compound ist einzigartig in seinem gut dokumentierten Sicherheitsprofil und seiner breiten Palette an Anwendungen, was es zu einer vielseitigen Verbindung sowohl in der Forschung als auch in klinischen Umgebungen macht.
Eigenschaften
IUPAC Name |
2-(2-oxopyrrolidin-1-yl)acetamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C6H10N2O2/c7-5(9)4-8-3-1-2-6(8)10/h1-4H2,(H2,7,9) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
GMZVRMREEHBGGF-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1CC(=O)N(C1)CC(=O)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C6H10N2O2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID5044491 | |
| Record name | 2-Oxo-1-Pyrrolidineacetamide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5044491 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
142.16 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Boiling Point |
Decomposes | |
| Record name | Piracetam | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09210 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Mechanism of Action |
Piracetam interacts with the polar heads in the phospholipids membrane and the resulting mobile drug-lipid complexes are thought to reorganize the lipids and influence membrane function and fluidity. Such interaction has been reported in a study that investigated the effects of neuronal outgrowth induced by beta amyloid peptides; while amyloid peptides cause lipid disorganization within the cell membranes leading to neuronal death, piracetam demonstrated to decrease the destabilizing effects of amyloid peptide. The authors suggest that piracetam induces a positive curvature of the membrane by occupying the polar groups in the phospholipids to counteract the negative curvature induced by amyloid peptides , which in turn would decrease the likelihood of membrane fusion. This mechanism of action is thought to improve membrane stability, allowing the membrane and transmembrane proteins to maintain and recover the three-dimensional structure or folding for normal function such as membrane transport, chemical secretion, and receptor binding and stimulation. Through restored membrane fluidity, piracetam promotes restored neurotransmission such as glutamatergic and cholinergic systems, enhances neuroplasticity and mediates neuroprotective and anticonvulsant effects at the neuronal level. It is also demonstrated that piracetam also improves the fluidity of platelet membranes. At the vascular level, piracetam decreases adhesion of erythrocytes to cell wall and reduces vasospasm which in turn improves microcirculation including cerebral and renal blood flow., It was found that a drug of the nootropic nature piracetam possessing pronounced antihypoxic properties eliminates calcium chloride-induced disturbances of the cardiac rhythm and significantly raises the threshold of atrial fibrillation during electrical stimulation. The drug's antiarrhythmic effect is followed by a decrease of the rhythm rate and an increase of the contraction amplitude. The animals treated with piracetam in a dose when its antiarrhythmic effects (300 mg/kg) exhibited a decrease of the membrane potential of erythrocytes as compared with control. Similar effects occurred in the animals treated with lidocaine. It can be concluded that in certain types of arrhythmias the use of piracetam restores the normal rhythm of contractions that is perhaps connected with its positive influence on metabolic processes in the myocardium. | |
| Record name | Piracetam | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09210 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | PIRACETAM | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7529 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Crystals from isopropanol | |
CAS No. |
7491-74-9 | |
| Record name | Piracetam | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=7491-74-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Piracetam [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0007491749 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Piracetam | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09210 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | piracetam | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=758191 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | 2-Oxo-1-Pyrrolidineacetamide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5044491 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Piracetam | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.028.466 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | PIRACETAM | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/ZH516LNZ10 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | PIRACETAM | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7529 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Melting Point |
151.5 - 152.5 °C | |
| Record name | Piracetam | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09210 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | PIRACETAM | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7529 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.


![(2E,4E)-2-cyano-5-[5-(2,5-dichlorophenyl)furan-2-yl]penta-2,4-dienethioamide](/img/structure/B1677875.png)