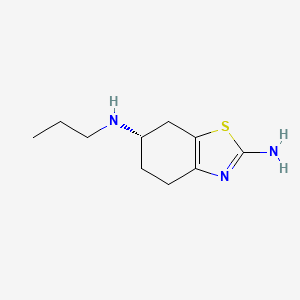
Pramipexol
Übersicht
Beschreibung
Pramipexole is a medication primarily used to treat Parkinson’s disease and restless legs syndrome. It is a non-ergot dopamine agonist that mimics the activity of dopamine, a neurotransmitter in the brain. Pramipexole was first approved for medical use in the United States in 1997 .
Wirkmechanismus
Target of Action
Pramipexole is a non-ergot dopamine agonist . It has a high relative in vitro specificity and full intrinsic activity at the D2 subfamily of dopamine receptors, binding with higher affinity to D3 than to D2 or D4 receptor subtypes . The relevance of D3 receptor binding in Parkinson’s disease is unknown .
Mode of Action
Pramipexole works by acting in place of dopamine, a natural substance in the brain that is needed to control movement . In normal dopaminergic systems, pramipexole acts on presynaptic D2 and D3 dopamine autoreceptors and suppresses the synthesis and synaptic release of dopamine . Effects on postsynaptic receptors are elicited only at higher dose levels and with substantially longer latencies than are needed for presynaptic autoreceptor stimulation .
Biochemical Pathways
Pramipexole modulates fronto-subthalamic or fronto-striatal pathways during sequential working memory . It also prevents ischemic cell death via mitochondrial pathways in ischemic stroke . Pramipexole elevated the mitochondrial membrane potential and mitochondrial oxidative phosphorylation .
Pharmacokinetics
It is excreted to the kidney . It is promptly absorbed, reaching high plasma concentrations within 1–2 hours, with a half-life of 6–8 hours . In patients with renal impairment, clearance of pramipexole is about 75% lower in patients with Cl cr of approximately 20 mL/minute and about 60% lower in patients with Cl cr of approximately 40 mL/minute .
Result of Action
Pramipexole promotes the neurological recovery as shown by the panel of neurobehavioral tests . Post-stroke treatment with pramipexole reduced levels of mitochondrial ROS and Ca 2+ after ischemia . Pramipexole inhibited the transfer of cytochrome c from mitochondria to cytosol, and hence inhibited the mitochondrial permeability transition pore .
Action Environment
The efficacy of pramipexole can be influenced by various environmental factors. For instance, a trend toward improvement in quality of life was consistently observed among patients who received optimal doses of pramipexole (≥80% of the study population on 1.5 mg dosage), regardless of disease severity (advanced versus early) or baseline quality of life levels . This suggests that the dosage, disease severity, and baseline health status of the patient can all influence the action and efficacy of pramipexole.
Wissenschaftliche Forschungsanwendungen
Pramipexol hat eine breite Palette von Anwendungen in der wissenschaftlichen Forschung:
Chemie: Wird als Modellverbindung in Studien über Dopaminagonisten verwendet.
Biologie: Untersucht auf seine Auswirkungen auf Dopaminrezeptoren und Neurotransmission.
Medizin: Weit verbreitet in der Behandlung der Parkinson-Krankheit und des Restless-Legs-Syndroms. .
Industrie: Eingesetzt bei der Entwicklung neuer Pharmazeutika, die auf Dopaminrezeptoren abzielen.
5. Wirkmechanismus
This compound entfaltet seine Wirkung, indem es Dopaminrezeptoren im Gehirn stimuliert, insbesondere die D2-, D3- und D4-Rezeptoren. Durch die Bindung an diese Rezeptoren verstärkt this compound die Dopaminaktivität, was zur Linderung der Symptome der Parkinson-Krankheit und des Restless-Legs-Syndroms beiträgt. Der genaue Wirkmechanismus ist noch nicht vollständig geklärt, es wird jedoch angenommen, dass er die Modulation der Neurotransmitterfreisetzung und neuronaler Signalwege beinhaltet .
Ähnliche Verbindungen:
Ropinirol: Ein weiterer nicht-ergotischer Dopaminagonist, der zur Behandlung der Parkinson-Krankheit und des Restless-Legs-Syndroms eingesetzt wird.
Rotigotin: Ein Dopaminagonist, der als transdermales Pflaster zur Behandlung der Parkinson-Krankheit und des Restless-Legs-Syndroms erhältlich ist.
Apomorphin: Ein Dopaminagonist, der zur Behandlung der Parkinson-Krankheit eingesetzt wird, insbesondere zur Behandlung von „Off“-Episoden.
Eindeutigkeit von this compound: this compound ist einzigartig aufgrund seiner hohen Affinität zum D3-Rezeptor, was zu seiner Wirksamkeit bei der Behandlung der Parkinson-Krankheit und des Restless-Legs-Syndroms beitragen soll. Darüber hinaus hat sich gezeigt, dass this compound neuroprotektive und antioxidative Eigenschaften besitzt, was es zu einem vielversprechenden Kandidaten für weitere Forschungen zu neurodegenerativen Erkrankungen macht .
Biochemische Analyse
Biochemical Properties
Pramipexole interacts with dopamine receptors, specifically the D2, D3, and D4 subtypes . By binding to these receptors, pramipexole stimulates dopamine activity in the brain, which helps alleviate symptoms of Parkinson’s disease and RLS. The compound has a high affinity for the D3 receptor, which is believed to contribute to its efficacy in treating these conditions .
Cellular Effects
Pramipexole influences various cellular processes, including cell signaling pathways, gene expression, and cellular metabolism. It has been shown to increase dopamine signaling in neurons, which helps restore normal motor function in patients with Parkinson’s disease . Additionally, pramipexole has neuroprotective effects, potentially due to its antioxidant properties and ability to stabilize mitochondria .
Molecular Mechanism
At the molecular level, pramipexole acts as an agonist for dopamine receptors, particularly the D2, D3, and D4 subtypes . By binding to these receptors, pramipexole stimulates dopamine activity in the brain, which helps alleviate symptoms of Parkinson’s disease and RLS. The compound also inhibits the release of somatostatin, a hormone that regulates the endocrine system .
Temporal Effects in Laboratory Settings
In laboratory settings, pramipexole has been shown to have stable effects over time. Studies have demonstrated that the compound maintains its efficacy in reducing symptoms of Parkinson’s disease and RLS over extended periods . Additionally, pramipexole has been found to have a long half-life, which contributes to its sustained effects .
Dosage Effects in Animal Models
In animal models, pramipexole has been shown to have dose-dependent effects. Lower doses of the compound are effective in alleviating symptoms of Parkinson’s disease and RLS, while higher doses can lead to adverse effects such as nausea, dizziness, and hallucinations . The optimal dosage of pramipexole varies depending on the specific condition being treated and the individual patient’s response to the medication .
Metabolic Pathways
Pramipexole undergoes minimal metabolism in the human body, with the majority of the compound being excreted unchanged in the urine . This limited metabolism reduces the risk of drug interactions and makes pramipexole a relatively safe option for long-term use. The primary route of elimination is through the kidneys, with approximately 90% of the drug being excreted in the urine .
Transport and Distribution
Pramipexole is extensively distributed throughout the body, with a volume of distribution of approximately 500 liters . The compound is about 15% bound to plasma proteins, which allows it to be readily available for interaction with dopamine receptors . Pramipexole is also distributed into red blood cells, with an erythrocyte-to-plasma ratio of approximately 2 .
Subcellular Localization
Pramipexole primarily localizes to the mitochondria within cells, where it exerts its neuroprotective effects by stabilizing mitochondrial function and reducing oxidative stress . This subcellular localization is crucial for the compound’s ability to protect neurons from damage and maintain normal cellular function.
Vorbereitungsmethoden
Synthetic Routes and Reaction Conditions: The synthesis of pramipexole involves the reaction of (S)-2,6-diamino-4,5,6,7-tetrahydrobenzothiazole with an alkylating agent. This reaction is carried out in the absence of a base, in a solvent from which the resulting N-monoalkylated product selectively precipitates out as a salt. The salt is then treated with an inorganic base to convert it into the free pramipexole base, which can be further converted into pharmaceutically acceptable salts .
Industrial Production Methods: In industrial settings, pramipexole is produced using a multi-step process. Starting with 4-aminocyclohexanol, the compound undergoes acylation, oxidation, alpha-halogenation, ring closing, hydrolysis, resolution, propionylation, and reduction reactions to obtain pramipexole. The final product can be converted into pramipexole hydrochloride through a salt-forming reaction with hydrochloric acid .
Analyse Chemischer Reaktionen
Arten von Reaktionen: Pramipexol unterliegt verschiedenen chemischen Reaktionen, darunter:
Oxidation: Umfasst die Verwendung von Oxidationsmitteln wie Trichlorisocyanursäure.
Reduktion: Verwendet Reduktionsmittel wie Natriumborhydrid und Boran.
Substitution: Umfasst den Austausch von funktionellen Gruppen innerhalb des Moleküls.
Häufige Reagenzien und Bedingungen:
Oxidation: Trichlorisocyanursäure wird als Oxidationsmittel verwendet.
Reduktion: Natriumborhydrid und Boran sind übliche Reduktionsmittel.
Substitution: Verschiedene Alkylierungsmittel werden für Substitutionsreaktionen verwendet.
Hauptprodukte: Die Hauptprodukte, die aus diesen Reaktionen entstehen, umfassen Pramipexolbase und ihre pharmazeutisch verträglichen Salze, wie z. B. Pramipexolhydrochlorid .
Vergleich Mit ähnlichen Verbindungen
Ropinirole: Another non-ergot dopamine agonist used to treat Parkinson’s disease and restless legs syndrome.
Rotigotine: A dopamine agonist available as a transdermal patch for the treatment of Parkinson’s disease and restless legs syndrome.
Apomorphine: A dopamine agonist used for the treatment of Parkinson’s disease, particularly for managing “off” episodes.
Uniqueness of Pramipexole: Pramipexole is unique due to its high affinity for the D3 receptor, which is thought to contribute to its efficacy in treating Parkinson’s disease and restless legs syndrome. Additionally, pramipexole has been shown to have neuroprotective and antioxidant properties, making it a promising candidate for further research in neurodegenerative diseases .
Eigenschaften
IUPAC Name |
(6S)-6-N-propyl-4,5,6,7-tetrahydro-1,3-benzothiazole-2,6-diamine | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C10H17N3S/c1-2-5-12-7-3-4-8-9(6-7)14-10(11)13-8/h7,12H,2-6H2,1H3,(H2,11,13)/t7-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
FASDKYOPVNHBLU-ZETCQYMHSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCCNC1CCC2=C(C1)SC(=N2)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CCCN[C@H]1CCC2=C(C1)SC(=N2)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C10H17N3S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID6023496 | |
| Record name | Pramipexole | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID6023496 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
211.33 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Pramipexole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014557 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
freely soluble in water, 1.40e-01 g/L | |
| Record name | Pramipexole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00413 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Pramipexole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014557 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
The exact mechanism of action of pramipexole as a treatment for Parkinson's disease is unknown at this time. It is thought, however, that the ability of pramipexole to cause stimulation of the dopamine receptors in the striatum of the brain, a region that receives a vast array of neurological input and is responsible for a wide variety of functions, may be involved. Studies performed in animals show that pramipexole influences striatal neuronal transmission rates following activation of dopamine receptors. Pramipexole is considered a non-ergot dopamine agonist that shows specificity and strong activity at the D2 subfamily of dopamine receptors in vitro, binding selectively and dopamine D2 receptors and showing a preference for the dopamine D3 receptor subtype rather than other subtypes. The clinical significance of this binding specificity is unknown,., The purpose of this study was to determine the binding sites of pramipexole in extrastriatal dopaminergic regions because its antidepressive effects have been speculated to occur by activating the dopamine D(2) receptor subfamily in extrastriatal areas. Dynamic positron emission tomography (PET) scanning using (11)C-FLB 457 for quantification of D(2)/D(3) receptor subtype was performed on 15 healthy volunteers. Each subject underwent two PET scans before and after receiving a single dose of pramipexole (0, 0.125, or 0.25 mg). The study demonstrated that pramipexole significantly binds to D(2)/D(3) receptors in the prefrontal cortex, amygdala, and medial and lateral thalamus at a dose of 0.25 mg. These regions have been indicated to have some relation to depression and may be part of the target sites where pramipexole exerts its antidepressive effects., Pramipexole dihydrochloride, a synthetic benzothiazolamine derivative, is a nonergot-derivative dopamine receptor agonist. In in vitro binding studies, pramipexole demonstrated high binding specificity for and intrinsic activity at dopamine D2 receptors compared with other dopamine receptor agonists (e.g., bromocriptine, pergolide), having a higher affinity for the D3 receptor subtype than for the D2 or D4 subtypes. Pramipexole binds with moderate affinity to alpha2-adrenergic receptors but has little or no affinity for alpha1- or beta-adrenergic, acetylcholine, dopamine D1, or serotonin (5-hydroxytryptamine (5-HT)) receptors., Our aim was to determine if pramipexole, a D3 preferring agonist, effectively reduced dopamine neuron and fiber loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model when given at intraperitoneal doses corresponding to clinical doses. We also determined whether subchronic treatment with pramipexole regulates dopamine transporter function, thereby reducing intracellular transport of the active metabolite of MPTP, 1-methyl-4-phenylpyridinium (MPP+). Ten 12-month old C57BL/6 mice were treated with MPTP (or saline) twice per day at 20 mg/kg s.c. (4 injections over 48 h). Mice were pretreated for 3 days and during the 2-day MPTP regimen with pramipexole (0.1 mg/kg/day) or saline. Stereological quantification of dopamine neuron number and optical density measurement of dopamine fiber loss were carried out at 1 week after treatment, using immunostaining for dopamine transporter (DAT) and tyrosine hydroxylase (TH). Additional wild-type (WT) and D3 receptor knockout (KO) mice were treated for 5 days with pramipexole (0.1 mg/kg/day) or vehicle. The kinetics of (3)H-MPP+ and (3)H-DA uptake (Vmax and Km) were determined 24 hr later; and at 24 hr and 14 days dopamine transporter density was measured by quantitative autoradiography. Pramipexole treatment completely antagonized the neurotoxic effects of MPTP, as measured by substantia nigra and ventral tegmental area TH-immunoreactive cell counts. MPTP- induced loss of striatal innervation, as measured by DAT-immunoreactivity, was partially prevented by pramipexole, but not with regard to TH-IR. Pramipexole also reduced DAT- immunoreactivity in non-MPTP treated mice. Subchronic treatment with pramipexole lowered the Vmax for (3)H-DA and (3)H-MPP+ uptake into striatal synaptosomes of WT mice. Pramipexole treatment lowered Vmax in WT but not D3 KO mice; however, D3 KO mice had lower Vmax for (3)H-DA uptake. There was no change in DAT number in WT with pramipexole treatment or D3 KO mice at 24 hr post-treatment, but there was a reduction in WT-pramipexole treated and not in D3 KO mice at 14 days post-treatment. These results suggest that protection occurs at clinically suitable doses of pramipexole. Protection could be due to a reduced amount of MPP+ taken up into DA terminals via DAT. D3 receptor plays an important role in this regulation of transporter uptake and availability. | |
| Record name | Pramipexole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00413 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | PRAMIPEXOLE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8253 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
CAS No. |
104632-26-0 | |
| Record name | Pramipexole | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=104632-26-0 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Pramipexole [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0104632260 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Pramipexole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00413 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Pramipexole | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID6023496 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | (S)-2-Amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.124.761 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | PRAMIPEXOLE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/83619PEU5T | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | PRAMIPEXOLE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8253 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Pramipexole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014557 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
288-290 | |
| Record name | Pramipexole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00413 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Synthesis routes and methods
Procedure details





Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.
![4-Amino-1-phenylpyrazolo[3,4-d]pyrimidine](/img/structure/B1677961.png)
![1-Chloro-4-[2-chloro-1-(4-chlorophenyl)ethyl]benzene](/img/structure/B1677968.png)
![13-(2-Fluorophenyl)-3,5,10,10-tetramethyl-8-phenyl-12-oxa-3,5,9-triazatricyclo[7.4.0.02,7]trideca-1,7-diene-4,6-dione](/img/structure/B1677977.png)
![2-[4-(2,3-dimethylphenyl)piperazin-1-yl]-N-(2-ethoxyphenyl)acetamide](/img/structure/B1677980.png)
