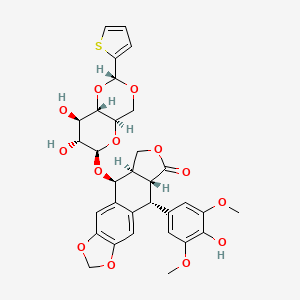
Teniposide
Übersicht
Beschreibung
Wirkmechanismus
Target of Action
Teniposide’s primary target is DNA topoisomerase II , an enzyme that aids in DNA unwinding . This enzyme plays a crucial role in various aspects of DNA metabolism, including replication, transcription, and recombination .
Mode of Action
This compound inhibits DNA synthesis by forming a complex with topoisomerase II and DNA . This complex induces breaks in double-stranded DNA and prevents repair by topoisomerase II binding .
Biochemical Pathways
The formation of the this compound-topoisomerase II-DNA complex and the subsequent DNA breaks disrupt the normal biochemical pathways of the cell. This disruption primarily affects the cell cycle, preventing cells from entering the mitotic phase . This compound acts primarily in the G2 and S phases of the cell cycle .
Pharmacokinetics
This compound is eliminated biphasically, with a terminal half-life of 5 hours . The pharmacokinetics of this compound is linear at doses up to 1000 mg/m² . The oral bioavailability is about 40% . About 45% of a radiolabelled dose of this compound is excreted in the urine, with 4-14% occurring as the parent drug .
Result of Action
The result of this compound’s action is the induction of cell death . The drug causes dose-dependent single- and double-stranded breaks in DNA and DNA-protein cross-links . These disruptions prevent cells from entering the mitotic phase of the cell cycle, leading to cell death .
Action Environment
The action of this compound can be influenced by the environment within the body. For example, the drug is more highly protein-bound than its analog etoposide, and its uptake and binding to cells is also greater . Additionally, the drug’s action can be affected by the presence of other drugs, as well as the patient’s overall health status .
Wissenschaftliche Forschungsanwendungen
Teniposide hat eine breite Palette von Anwendungen in der wissenschaftlichen Forschung:
Chemie: Als Modellverbindung verwendet, um die Mechanismen der Topoisomerase-II-Hemmung zu untersuchen.
Biologie: Untersucht wegen seiner Auswirkungen auf die Zellzyklusregulation und Apoptose.
Industrie: In der Entwicklung neuer Chemotherapeutika und Medikamententrägersysteme verwendet.
5. Wirkmechanismus
This compound übt seine Wirkung aus, indem es das Enzym Topoisomerase II hemmt, das für die DNA-Replikation und Zellteilung unerlässlich ist . Durch die Bildung eines Komplexes mit Topoisomerase II und DNA induziert this compound Brüche in doppelsträngiger DNA und verhindert die Reparatur, was zum Zelltod führt . Dieser Mechanismus betrifft hauptsächlich Zellen in der G2- und S-Phase des Zellzyklus .
Ähnliche Verbindungen:
Etoposide: Ein weiteres Podophyllotoxin-Derivat mit ähnlichem Wirkmechanismus.
Podophyllotoxin: Die Stammverbindung, von der this compound abgeleitet ist.
Vergleich:
Etoposide vs. This compound: Beide Verbindungen hemmen die Topoisomerase II, aber this compound hat eine stärkere Penetration des zentralen Nervensystems und ist lipophiler.
Podophyllotoxin vs. This compound: Während Podophyllotoxin der natürliche Vorläufer ist, wird this compound chemisch modifiziert, um seine therapeutische Wirksamkeit zu verbessern und die Toxizität zu reduzieren.
Die einzigartigen Eigenschaften von this compound, wie seine höhere Lipophilie und größere Penetration des zentralen Nervensystems, machen es zu einem wertvollen Chemotherapeutikum mit eindeutigen Vorteilen gegenüber ähnlichen Verbindungen .
Biochemische Analyse
Biochemical Properties
Teniposide plays a significant role in biochemical reactions. It interacts with topoisomerase II, a crucial enzyme involved in DNA replication and transcription . The interaction between this compound and topoisomerase II is complex, leading to breaks in double-stranded DNA and preventing repair by topoisomerase II binding .
Cellular Effects
This compound has profound effects on various types of cells and cellular processes. It influences cell function by inducing breaks in double-stranded DNA, which can impact cell signaling pathways, gene expression, and cellular metabolism .
Molecular Mechanism
The mechanism of action of this compound involves forming a complex with topoisomerase II and DNA . This complex induces breaks in double-stranded DNA and prevents repair by topoisomerase II binding . This action at the molecular level can lead to changes in gene expression and potentially result in cell death, particularly in cancer cells .
Metabolic Pathways
This compound is involved in the metabolic pathway related to DNA synthesis . It interacts with the enzyme topoisomerase II during this process .
Transport and Distribution
Given its mechanism of action, it’s likely that it interacts with transporters or binding proteins involved in DNA synthesis and repair .
Subcellular Localization
Given its interaction with topoisomerase II, it’s likely localized to areas of the cell where DNA synthesis and repair occur .
Vorbereitungsmethoden
Synthetic Routes and Reaction Conditions: Teniposide is a semisynthetic derivative of podophyllotoxin. The synthesis involves the glycosylation of podophyllotoxin with a D-glucose derivative . The reaction conditions typically include the use of acidic or basic catalysts to facilitate the glycosylation process.
Industrial Production Methods: Industrial production of this compound involves large-scale extraction of podophyllotoxin from the rhizomes of the wild mandrake (Podophyllum peltatum), followed by chemical modification to produce this compound . The process is optimized to ensure high yield and purity of the final product.
Analyse Chemischer Reaktionen
Arten von Reaktionen: Teniposide unterliegt verschiedenen chemischen Reaktionen, darunter:
Oxidation: this compound kann oxidiert werden, um reaktive Zwischenprodukte zu bilden.
Reduktion: Reduktionsreaktionen können die funktionellen Gruppen an this compound verändern.
Substitution: Substitutionsreaktionen können an bestimmten Stellen im this compound-Molekül auftreten.
Häufige Reagenzien und Bedingungen:
Oxidationsmittel: Wasserstoffperoxid, Kaliumpermanganat.
Reduktionsmittel: Natriumborhydrid, Lithiumaluminiumhydrid.
Substitutionsreagenzien: Halogene, Nukleophile.
Hauptprodukte: Die Hauptprodukte, die aus diesen Reaktionen gebildet werden, hängen von den verwendeten Reagenzien und Bedingungen ab. Beispielsweise kann Oxidation zur Bildung von Chinonen führen, während Reduktion Alkohole oder Amine ergeben kann.
Vergleich Mit ähnlichen Verbindungen
Etoposide: Another podophyllotoxin derivative with a similar mechanism of action.
Podophyllotoxin: The parent compound from which teniposide is derived.
Comparison:
Etoposide vs. This compound: Both compounds inhibit topoisomerase II, but this compound has greater central nervous system penetrance and is more lipophilic.
Podophyllotoxin vs. This compound: While podophyllotoxin is the natural precursor, this compound is chemically modified to improve its therapeutic efficacy and reduce toxicity.
This compound’s unique properties, such as its higher lipophilicity and greater central nervous system penetrance, make it a valuable chemotherapeutic agent with distinct advantages over similar compounds .
Eigenschaften
IUPAC Name |
(5S,5aR,8aR,9R)-5-[[(2R,4aR,6R,7R,8R,8aS)-7,8-dihydroxy-2-thiophen-2-yl-4,4a,6,7,8,8a-hexahydropyrano[3,2-d][1,3]dioxin-6-yl]oxy]-9-(4-hydroxy-3,5-dimethoxyphenyl)-5a,6,8a,9-tetrahydro-5H-[2]benzofuro[6,5-f][1,3]benzodioxol-8-one | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C32H32O13S/c1-37-19-6-13(7-20(38-2)25(19)33)23-14-8-17-18(42-12-41-17)9-15(14)28(16-10-39-30(36)24(16)23)44-32-27(35)26(34)29-21(43-32)11-40-31(45-29)22-4-3-5-46-22/h3-9,16,21,23-24,26-29,31-35H,10-12H2,1-2H3/t16-,21+,23+,24-,26+,27+,28+,29+,31+,32-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
NRUKOCRGYNPUPR-QBPJDGROSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
COC1=CC(=CC(=C1O)OC)C2C3C(COC3=O)C(C4=CC5=C(C=C24)OCO5)OC6C(C(C7C(O6)COC(O7)C8=CC=CS8)O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
COC1=CC(=CC(=C1O)OC)[C@H]2[C@@H]3[C@H](COC3=O)[C@@H](C4=CC5=C(C=C24)OCO5)O[C@H]6[C@@H]([C@H]([C@H]7[C@H](O6)CO[C@H](O7)C8=CC=CS8)O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C32H32O13S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID8023638 | |
| Record name | Teniposide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8023638 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
656.7 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
Practically insoluble in water., In water, 5.9 mg/L at 25 °C /Estimated/ | |
| Record name | TENIPOSIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6546 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Vapor Pressure |
6.8X10-26 mm Hg at 25 °C /Estimated/ | |
| Record name | TENIPOSIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6546 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Mechanism of Action |
The mechanism of action appears to be related to the inhibition of type II topoisomerase activity since teniposide does not intercalate into DNA or bind strongly to DNA. Teniposide binds to and inhibits DNA topoisomerase II. The cytotoxic effects of teniposide are related to the relative number of double-stranded DNA breaks produced in cells, which are a reflection of the stabilization of a topoisomerase II-DNA intermediate., It is an inhibitor of DNA topoisomerase II enzymes: Teniposide is a DNA topoisomerase II poison that has been shown to promote DNA cleavage, with a strong preference for a C or T at position -1. Most of the mutational events reported in mammalian cells, including point mutations, chromosomal deletions and exchanges and aneuploidy, can be explained by this activity. Teniposide does not inhibit bacterial topoisomerases and may not mutate bacterial cells by the same mechanism as mammalian cells. Unlike many other DNA topoisomerase II poisons, teniposide does not bind to DNA, either covalently or by intercalation. Instead, it appears to interact directly with the DNA topoisomerase II enzyme., ... The drug appears to produce its cytotoxic effects by damaging DNA and thereby inhibiting or altering DNA synthesis. Teniposide has been shown to induce single-stranded DNA breaks; the drug also induces double-stranded DNA breaks and DNA-protein cross links. ... Teniposide appears to be cell cycle specific, inducing G2-phase arrest and preferentially killing cells in the G2 and late S phases. | |
| Record name | Teniposide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00444 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | TENIPOSIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6546 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Impurities |
... The 50-mg intravenous preparation typically also contains benzyl alcohol (0.15 g), N,N-dimethylacetamide (0.3 g), polyethoxylated castor oil (2.5 g), maleic acid to a pH of 5.1 and absolute ethanol to 5 mL. | |
| Record name | TENIPOSIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6546 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Crystals from absolute ethanol | |
CAS No. |
29767-20-2 | |
| Record name | Teniposide | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=29767-20-2 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Teniposide [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0029767202 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Teniposide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00444 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Teniposide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8023638 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Teniposide | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.045.286 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | TENIPOSIDE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/957E6438QA | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | TENIPOSIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6546 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Melting Point |
242-246 °C | |
| Record name | Teniposide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00444 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | TENIPOSIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6546 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.


![4-(4-Chloroanilino)-2-[4-[(2E)-2-hydroxyiminopropanoyl]piperazin-1-yl]-6-propan-2-yl-5H-pyrrolo[3,4-d]pyrimidin-7-one](/img/structure/B1684410.png)
![N-[(2S)-2-(3,4-dichlorophenyl)-4-[4-(2-oxo-1,3-diazinan-1-yl)piperidin-1-yl]butyl]-N-methylbenzamide](/img/structure/B1684412.png)
![1,1-dimethyl-3-[(E)-1-pyridin-2-ylethylideneamino]thiourea](/img/structure/B1684415.png)
![3-[2-(2-Cyclopentyl-6-{[4-(dimethylphosphoryl)phenyl]amino}-9H-purin-9-YL)ethyl]phenol](/img/structure/B1684423.png)