Carboplatin
Übersicht
Beschreibung
Carboplatin is a platinum-based chemotherapy medication used to treat various forms of cancer, including ovarian cancer, lung cancer, head and neck cancer, brain cancer, and neuroblastoma . It was developed as a less toxic analogue of cisplatin and was approved for medical use in 1989 . This compound works by interfering with the duplication of DNA, thereby inhibiting the growth of cancer cells .
Wirkmechanismus
Target of Action
Carboplatin, also known as Paraplatin, is an organoplatinum antineoplastic alkylating agent . Its primary targets are the nucleotides in the DNA of cancer cells . By attaching alkyl groups to these nucleotides, it interferes with the DNA structure and inhibits DNA synthesis .
Mode of Action
This compound forms intra- and inter-strand crosslinks within the cell . This modifies the DNA structure and stops DNA synthesis . The compound predominantly acts by leading to the formation of monoadducts, and DNA fragmenting when repair enzymes attempt to correct the error . About 2% of this compound’s activity comes from DNA cross-linking from a base on one strand to a base on another, preventing DNA strands from separating .
Biochemical Pathways
This compound affects several biochemical pathways. It modulates STAT signaling and induces an immunogenic type of cancer cell death through exposure of calreticulin and release of ATP and high-mobility group protein box-1 (HMGB-1) . It also enhances the effector immune response through modulation of programmed death receptor 1-ligand and mannose-6-phosphate receptor expression .
Pharmacokinetics
Its clearance is proportional to the glomerular filtration rate and the volume of distribution of the central compartment appears to correlate with extracellular fluid volume . The elimination half-life varies with renal function and is typically between 2 and 6 hours in patients with a normal glomerular filtration rate and may be as long as 18 hours in patients with impaired renal function .
Result of Action
This compound stops or slows the growth of cancer cells and other rapidly growing cells by damaging their DNA . It is used to treat a number of forms of cancer, including advanced ovarian cancer, lung cancer, head and neck cancer, brain cancer, and neuroblastoma . The main drawback of this compound is its myelosuppressive effect. This causes the blood cell and platelet output of bone marrow in the body to decrease quite dramatically, sometimes as low as 10% of its usual production levels .
Action Environment
The action, efficacy, and stability of this compound are influenced by various environmental factors. For instance, the renal function of the patient plays a significant role in the clearance of the drug . Furthermore, the expression of certain genes like ERCC1 can impact the outcomes of platinum-based treatment . Understanding these factors can help optimize the use of this compound in cancer treatment.
Wissenschaftliche Forschungsanwendungen
Carboplatin hat eine breite Palette von Anwendungen in der wissenschaftlichen Forschung:
5. Wirkmechanismus
This compound entfaltet seine Wirkung, indem es reaktive Platinkomplexe bildet, die innerhalb der Zelle zu intra- und interstrangartigen Quervernetzungen von DNA-Molekülen führen . Diese Modifikation der DNA-Struktur hemmt die DNA-Synthese und beeinflusst den Zellzyklus, was letztendlich zum Zelltod führt . Die molekularen Zielstrukturen von this compound umfassen DNA, an die es kovalente Bindungen eingeht und den Replikationsprozess stört .
Ähnliche Verbindungen:
Cisplatin: Der Vorgänger von this compound, bekannt für seine höhere Toxizität, aber ähnlichen Wirkmechanismus.
Oxaliplatin: Ein weiteres platinbasiertes Chemotherapeutikum mit einem anderen Wirkungsspektrum und Toxizitätsprofil.
Vergleich:
Cisplatin vs. This compound: this compound ist weniger toxisch als Cisplatin, insbesondere in Bezug auf Nephrotoxizität und gastrointestinale Nebenwirkungen.
Oxaliplatin vs. This compound: Oxaliplatin hat ein anderes Wirkungsspektrum und wird häufig zur Behandlung von Darmkrebs eingesetzt.
Die reduzierte Toxizität und ähnliche Wirksamkeit von this compound machen es zu einer wertvollen Alternative zu Cisplatin in Chemotherapieschemata, insbesondere für Patienten, die die Nebenwirkungen von Cisplatin nicht vertragen .
Biochemische Analyse
Biochemical Properties
Carboplatin predominantly acts by attaching alkyl groups to the nucleotides, leading to the formation of monoadducts, and DNA fragmenting when repair enzymes attempt to correct the error . The copper transporter 1 seems to be of particular importance for this compound uptake in tumor cells .
Cellular Effects
This compound has a profound impact on various types of cells and cellular processes. It can cause a decrease in white blood cells (neutropenia) which can cause complications . Common side effects include low blood cell levels, nausea, and electrolyte problems .
Molecular Mechanism
This compound works by interfering with the duplication of DNA . It binds covalently to DNA and reacts with intrastrand and interstrand DNA sites to inhibit the production of proteins, while also inhibiting transcription .
Temporal Effects in Laboratory Settings
This compound has a myelosuppressive effect, causing the blood cell and platelet output of bone marrow in the body to decrease quite dramatically, sometimes as low as 10% of its usual production levels . The nadir of this myelosuppression usually occurs 21–28 days after the first treatment .
Dosage Effects in Animal Models
In animal models, a range of physiological and morphological effects was produced using doses calculated from the recommended therapeutic range (200–400 mg/m2) . A this compound dose reduction of as little as 10% was suggested to result in a doubling of 5-year relapse rate .
Metabolic Pathways
This compound is involved in metabolic pathways that lead to DNA damage. In the presence of glutathione S-transferase (GST), GSH combines with this compound to form a Pt-GSH complex that is excreted from cells by multidrug resistance-associated proteins (MRPs), consequently reducing intracellular this compound concentration .
Transport and Distribution
This compound is transported and distributed within cells and tissues through various transporters. The copper transporter 1 seems to be of particular importance for this compound uptake in tumor cells . This compound showed an obviously different pharmacokinetic characteristic from cisplatin and oxaliplatin. On the one hand, this compound has the highest proportion of Pt distribution in ultrafiltrate plasma .
Subcellular Localization
The distribution of total platinum in the cytosol is higher than in the mitochondria, followed by the nucleus . Enrichment of platinum in mitochondria may affect the respiratory chain or energy metabolism, and further lead to cell apoptosis .
Vorbereitungsmethoden
Synthesewege und Reaktionsbedingungen: Carboplatin wird durch die Reaktion von Cisplatin mit Cyclobutan-1,1-dicarbonsäure in Gegenwart von Ammoniak synthetisiert . Die Reaktionsbedingungen beinhalten typischerweise das Erhitzen des Gemisches, um die Bildung des Carboplatinkomplexes zu fördern.
Industrielle Produktionsverfahren: In industriellen Umgebungen wird this compound hergestellt, indem Cisplatin in Wasser gelöst und Cyclobutan-1,1-dicarbonsäure und Ammoniak hinzugefügt werden. Das Gemisch wird dann erhitzt, um die Reaktion zu fördern, und das entstandene this compound wird durch Kristallisation gereinigt .
Analyse Chemischer Reaktionen
Arten von Reaktionen: Carboplatin unterliegt verschiedenen chemischen Reaktionen, einschließlich Substitutionsreaktionen. Eine bemerkenswerte Reaktion ist die Aktivierung durch Chloridionen, die zur Entfernung des Malonatrestes und zur Bildung von Cisplatin führt .
Häufige Reagenzien und Bedingungen:
Chloridionen: Werden verwendet, um this compound zu aktivieren und in Cisplatin umzuwandeln.
Saure Bedingungen: Stark saure Umgebungen erleichtern die Umwandlung von this compound in Cisplatin.
Hauptprodukte, die gebildet werden:
Vergleich Mit ähnlichen Verbindungen
Cisplatin: The predecessor of carboplatin, known for its higher toxicity but similar mechanism of action.
Oxaliplatin: Another platinum-based chemotherapy drug with a different spectrum of activity and toxicity profile.
Comparison:
Cisplatin vs. This compound: this compound is less toxic than cisplatin, particularly in terms of nephrotoxicity and gastrointestinal side effects.
Oxaliplatin vs. This compound: Oxaliplatin has a different spectrum of activity and is often used to treat colorectal cancer.
This compound’s reduced toxicity and similar efficacy make it a valuable alternative to cisplatin in chemotherapy regimens, particularly for patients who cannot tolerate the side effects of cisplatin .
Eigenschaften
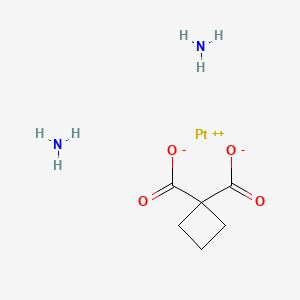
IUPAC Name |
azanide;cyclobutane-1,1-dicarboxylic acid;platinum(2+) | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C6H8O4.2H2N.Pt/c7-4(8)6(5(9)10)2-1-3-6;;;/h1-3H2,(H,7,8)(H,9,10);2*1H2;/q;2*-1;+2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
VSRXQHXAPYXROS-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1CC(C1)(C(=O)O)C(=O)O.[NH2-].[NH2-].[Pt+2] | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C6H12N2O4Pt | |
| Record name | Carboplatin | |
| Source | Wikipedia | |
| URL | https://en.wikipedia.org/wiki/Carboplatin | |
| Description | Chemical information link to Wikipedia. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Weight |
371.25 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
Sol in water, Water > 15 (mg/mL), pH 4 Acetate Buffer 5 - 10 (mg/mL), pH 9 Carbonate Buffer 5 - 10 (mg/mL), 10% Ethanol/H2O 5 - 10 (mg/mL), 95% Ethanol/H < 1 (mg/mL), 0.1NHC1 5 -10 (mg/mL), 0.1NNaOH 5 -10 (mg/mL), Methanol < 1 (mg/mL), Chloroform < 5 (mg/mL), Dimethylsulfoxide 5 (mg/mL), Acetic Acid < 1 (mg/mL), Trifluoroacetic Acid < 1 (mg/mL) | |
| Record name | CARBOPLATIN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6957 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | CARBOPLATIN | |
| Source | NCI Investigational Drugs | |
| URL | http://dtp.nci.nih.gov/NCI-InvestigationalDrugsCI92/241240%20(1992).txt | |
| Description | An investigational drug is one that is under study but does not have permission from the U.S. Food and Drug Administration (FDA) to be legally marketed and sold in the United States. NCI provides the investigational drug to the physicians who are participating in clinical trials or TRC protocols. For more information please visit NCI investigational drug website: https://www.cancer.gov/about-cancer/treatment/drugs/investigational-drug-access-fact-sheet | |
Color/Form |
White crystals | |
CAS No. |
41575-94-4 | |
| Record name | carboplatin | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=758182 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | carboplatin | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=241240 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | carboplatin | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=201345 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Diammine[cyclobutane-1,1-dicarboxylato(2-)-O,O']platinum | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.050.388 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | CARBOPLATIN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6957 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
A: Carboplatin, similar to cisplatin, exerts its cytotoxic effect by binding to DNA. [, , ] This binding primarily results in the formation of platinum-DNA adducts, specifically monoadducts and intra- and interstrand diadducts. [] These adducts interfere with DNA replication and transcription, ultimately triggering cell cycle arrest and apoptosis. [, ]
A: Yes, research using accelerator mass spectrometry has demonstrated that this compound forms DNA monoadducts at a rate approximately 100-fold slower than cisplatin. [] This difference in the kinetics of DNA adduct formation may contribute to the lower toxicity profile of this compound compared to cisplatin. []
A: Studies indicate that this compound can enhance the production and persistence of radiation-induced DNA single-strand breaks (SSBs). [] This effect was observed both when this compound was administered before irradiation under hypoxic conditions and when added after irradiation during the post-irradiation recovery period in air. [] This suggests a synergistic interaction between this compound and radiation therapy.
A: Research on human laryngeal squamous carcinoma cells (HN-3) showed that this compound treatment led to down-regulation of epidermal growth factor receptor (EGFR) expression. [] This effect was associated with increased reactive oxygen species (ROS) generation within the cells, indicating a potential mechanism for this compound's antitumor activity. []
A: Yes, computational studies have investigated the interaction between this compound and a B12N12 nano-cluster. [] This study utilized density functional theory (DFT) calculations with the B3P86 functional to explore the binding interactions and energy differences between various isomers of the this compound-nano-cluster complex. []
A: A study on a patient undergoing hemodialysis revealed a significantly lower area under the curve (AUC) of free platinum when this compound was administered shortly before hemodialysis. [] This suggests a substantial removal of this compound from the systemic circulation during hemodialysis, highlighting the importance of timing chemotherapy administration in this patient population.
A: In vitro experiments utilizing a bovine brain capillary endothelial cell model showed that hyperbaric oxygenation increased the transendothelial permeability of this compound. [] This suggests a potential strategy for enhancing this compound delivery to the brain, which is particularly relevant for treating brain tumors.
A: Several studies employed various cancer cell lines to investigate the in vitro efficacy of this compound, including human laryngeal squamous carcinoma cells (HN-3), [] three glioma cell lines (U251MG, SNB19, and LNZ308), [] two adenocarcinoma cell lines (MiaPaCa and SW480), [] five canine transitional cell carcinoma (TCC) cell lines, [] T24 human bladder cancer cells, [] COR-L23/P human non-small-cell lung carcinoma (NSCLC) cells, [] and two triple-negative breast cancer cell lines (CF41.Mg and MDA-MB-468). []
ANone: Several preclinical studies explored this compound-based combination therapies:
- This compound and Difluoromethylornithine (DFMO): Synergistic or additive effects were observed in a majority of the tested glioma and adenocarcinoma cell lines. []
- This compound and Vorinostat (SAHA): Synergy was noted in one glioma cell line and in the MiaPaCa cell line with short exposure times. []
- This compound and Docetaxel: Synergistic effects were observed in two out of three glioma cell lines and in the SW480 cell line with short exposures. []
- This compound and Gemcitabine: Synergistic activity at biologically achievable concentrations was observed in three out of five canine TCC cell lines. []
- This compound and XR5944: Enhanced anti-tumor activity was observed in COR-L23/P human non-small-cell lung carcinoma (NSCLC) xenograft models when XR5944 was administered before this compound. []
ANone: Numerous clinical trials have investigated the efficacy and safety of this compound in different cancer types:
- Head and Neck Cancer: A retrospective cohort study on US veterans with head and neck squamous cell carcinoma (HNSCC) suggested that this compound-based chemoradiotherapy was associated with improved overall survival compared to cetuximab-based chemoradiotherapy. [] This difference was particularly pronounced in patients with oropharynx cancer.
- A phase III randomized trial found that docetaxel-carboplatin was comparable to paclitaxel-carboplatin in terms of progression-free survival and response rate as a first-line chemotherapy regimen. [] Importantly, docetaxel-carboplatin was associated with significantly less neurotoxicity.
- A phase II trial explored the combination of gemcitabine/carboplatin with or without trastuzumab as first-line treatment for metastatic breast cancer. [] The regimen demonstrated promising activity, particularly in HER2-positive disease.
- A retrospective study from Japan evaluated high-dose ifosfamide, this compound, and etoposide combined with peripheral blood stem cell transplantation for male germ cell tumors. [] The treatment was found to be feasible and effective with manageable toxicity.
- A phase II trial evaluated the combination of vinorelbine and gemcitabine as first-line therapy for advanced NSCLC. [] The regimen showed promising activity and tolerability, comparable to standard platinum-based combinations.
- A phase II study investigated a triplet chemotherapy regimen of this compound, paclitaxel, and gemcitabine with amifostine support in advanced NSCLC. [] The combination demonstrated promising activity and good tolerability.
- A phase III trial compared nab-paclitaxel plus this compound to solvent-based paclitaxel plus this compound as first-line treatment for advanced NSCLC. [] Nab-paclitaxel plus this compound demonstrated a significantly improved overall response rate, particularly in patients with squamous cell histology.
- A phase II study assessed the feasibility, safety, and efficacy of this compound plus S-1 followed by maintenance S-1 therapy for patients with completely resected stage II/IIIA NSCLC. [] The study found that this regimen was not well-tolerated, highlighting the challenges of incorporating this compound into adjuvant settings for NSCLC.
- Glioblastoma: A phase 1/2 clinical trial investigated the feasibility and safety of blood-brain barrier (BBB) disruption using an implantable ultrasound system (SonoCloud-9) in combination with intravenous this compound in patients with recurrent glioblastoma. [] The study demonstrated the safety and feasibility of this approach, as well as a substantial increase in this compound concentration in the peritumoral brain region.
A: While considered less toxic than cisplatin, this compound can still cause side effects. Myelosuppression, particularly thrombocytopenia and neutropenia, is a common side effect of this compound treatment. [, , , , ] Other reported side effects include nausea, [] diarrhea, [] hepatotoxicity, [] nephrotoxicity, [] neurotoxicity, [] alopecia, [, , ] and hypersensitivity reactions. [, ] The severity and frequency of side effects can vary depending on the dose, schedule, and combination with other drugs.
A: One study explored the use of hyperbaric oxygenation as a method to enhance the transport of this compound across the blood-brain barrier. [] This approach showed promising results in vitro and warrants further investigation in clinical settings.
A: Research has explored the use of folate-targeted PEG as a potential carrier for this compound analogs. [] While the folate-targeted conjugates showed efficient cellular uptake via the folate receptor-mediated endocytosis pathway, they formed relatively few DNA adducts and exhibited higher IC50 values compared to this compound and non-targeted analogs. [] Further research is needed to optimize the design and delivery of targeted this compound conjugates.
ANone: Various analytical techniques have been used to investigate this compound, including:
- Accelerator Mass Spectrometry (AMS): Used to measure the kinetics of this compound-DNA adduct formation and drug uptake in cells. []
- Fluorescence-Based Microplate Cell Proliferation Assay: Employed to assess the effects of this compound on cell proliferation in vitro. []
- Propidium Iodide Staining: Used to evaluate cell cycle distribution in response to this compound treatment. []
- Caspase 3 and 7 Enzymatic Activity Measurement: Employed to assess apoptosis induction by this compound. []
- Atom Absorbing Spectrometry: Used to measure this compound concentration in axillary lymph nodes after regional injection of this compound-activated carbon suspension. []
- Immunofluorescence, Western Blotting, and qRT-PCR: Used to assess the expression levels of specific proteins and genes in response to this compound treatment. []
- Magnetic Resonance Imaging (MRI): Used to monitor tumor size and drug distribution in preclinical and clinical settings. [, ]
A: Research using a bovine brain capillary endothelial cell model suggests that this compound may be a substrate for P-glycoprotein (P-gp), a drug efflux transporter. [] The study demonstrated that verapamil, a P-gp inhibitor, enhanced the transendothelial permeability of this compound, similar to its effects on doxorubicin, a known P-gp substrate. [] This finding highlights the potential for drug-transporter interactions to influence this compound distribution and efficacy.
A: Yes, cisplatin is another platinum-based chemotherapeutic agent often used in cancer treatment. [, , ] While both drugs share a similar mechanism of action, they exhibit different pharmacokinetic and toxicity profiles. Clinical trials have directly compared this compound to cisplatin in various cancer types, such as NSCLC and ovarian cancer, with varying results. [, , ] The choice between this compound and cisplatin often depends on factors like tumor type, patient characteristics, and potential side effects.
A: Yes, research is ongoing to identify effective non-platinum-based chemotherapy options for various cancers. For instance, one study explored the combination of vinorelbine and gemcitabine as a potential alternative to platinum-containing regimens in advanced NSCLC. [] This combination demonstrated promising activity and a favorable toxicity profile compared to some platinum-based therapies.
ANone: The research on this compound exemplifies the collaborative nature of scientific advancements:
- Medicinal Chemistry: Plays a crucial role in designing and synthesizing this compound analogs with improved efficacy, pharmacokinetic properties, and reduced toxicity. []
- Pharmacology and Pharmaceutics: Focus on understanding the absorption, distribution, metabolism, and excretion (ADME) of this compound, as well as developing optimal drug delivery systems. [, ]
- Oncology and Clinical Research: Drive the evaluation of this compound in clinical trials, investigating its efficacy, safety, and optimal use in different cancer types and patient populations. [, , , , , , , , , , , , ]
- Molecular Biology and Genetics: Contribute to understanding the mechanisms of action, resistance, and potential biomarkers for this compound therapy. [, , , ]
- Analytical Chemistry: Develops and refines methods for characterizing this compound and monitoring its levels in biological samples. [, , ]
- Biomedical Engineering: Develops novel technologies for drug delivery, such as the implantable ultrasound system used to enhance this compound delivery to the brain. []
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.


![2-[[4-[(2-Amino-3-sulfanylpropyl)amino]-2-naphthalen-1-ylbenzoyl]amino]-4-methylpentanoic acid](/img/structure/B1684560.png)