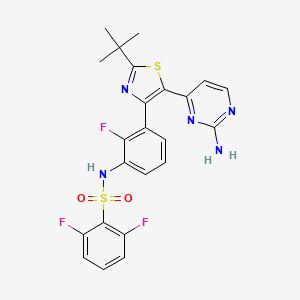
Dabrafenib
Übersicht
Beschreibung
Dabrafenib ist ein Kinase-Inhibitor, der hauptsächlich zur Behandlung von Krebserkrankungen eingesetzt wird, die mit Mutationen im BRAF-Gen zusammenhängen, wie z. B. Melanom, nicht-kleinzelliger Lungenkrebs und Schilddrüsenkrebs . Es wird unter dem Markennamen Tafinlar vermarktet und wurde erstmals im Mai 2013 in den Vereinigten Staaten für die medizinische Anwendung zugelassen .
Wirkmechanismus
Target of Action
Dabrafenib is a potent and selective inhibitor of some mutated forms of the protein kinase B-raf (BRAF) . The primary target of this compound is the BRAF V600E mutation, which is a serine/threonine protein kinase involved in cell growth .
Mode of Action
This compound works by binding to the ATP pocket of the BRAF enzyme, thereby inhibiting its activity . This inhibition is competitive and selective, with this compound showing a higher affinity for mutant forms of BRAF, including BRAF V600E, BRAF V600K, and BRAF V600D .
Biochemical Pathways
The primary biochemical pathway affected by this compound is the mitogen-activated protein kinase (MAPK) pathway . The BRAF mutations result in the activation of the MAPK pathway, which may promote tumor cell growth . By inhibiting BRAF, this compound disrupts this pathway, leading to a decrease in tumor cell proliferation .
Pharmacokinetics
The metabolism of this compound is primarily mediated by CYP2C8 and CYP3A4 to form hydroxy-dabrafenib . Hydroxy-dabrafenib is further oxidized via CYP3A4 to form carboxy-dabrafenib, which is subsequently excreted in bile and urine . Carboxy-dabrafenib is decarboxylated to form desmethyl-dabrafenib, which may be reabsorbed from the gut .
Result of Action
The molecular and cellular effects of this compound’s action include the regression and decreased proliferation of cells . By inhibiting the MAPK pathway in BRAF mutated cells, this compound leads to a decrease in tumor cell growth . This results in the regression of the cells and a decrease in their proliferation .
Action Environment
The action, efficacy, and stability of this compound can be influenced by various environmental factors. For instance, the presence of other drugs can affect the metabolism of this compound, potentially altering its efficacy . Additionally, factors such as the patient’s overall health, the presence of other diseases, and individual genetic factors can also influence the action of this compound .
Biochemische Analyse
Biochemical Properties
Dabrafenib acts as a competitive and selective BRAF inhibitor by binding to its ATP pocket . It has a higher affinity for mutant forms of BRAF, including BRAF V600E, BRAF V600K, and BRAF V600D . BRAF is a serine/threonine protein kinase and is involved in activating the RAS/MAPK pathway .
Cellular Effects
This compound has multifaceted influences on cells, particularly on migration at concentrations of 10 and 25 μM . It induces a decrease in cell viability, impedes cellular adhesion to the matrix, inhibits cellular aggregation and spheroid formation, arrests the cell cycle in the G1 phase, and induces apoptosis .
Molecular Mechanism
This compound inhibits the MAPK pathway by targeting the BRAF V600E mutation . This inhibition results in decreased MEK and ERK phosphorylation and inhibition of cell proliferation .
Temporal Effects in Laboratory Settings
In a BRAF V600E-containing xenograft model of human melanoma, orally administered this compound inhibited ERK activation, downregulated Ki67, and upregulated p27, leading to tumor growth inhibition . This compound also induced MAPK pathway activation in wild-type BRAF cells through CRAF (RAF1) signaling .
Dosage Effects in Animal Models
In a clinically relevant multidose cisplatin mouse model, this compound given orally twice daily with cisplatin showed protective effects as determined by functional hearing tests and cochlear outer hair cell counts . A this compound dose of 3 mg/kg BW, twice daily, in mice, was determined to be the minimum effective dose .
Metabolic Pathways
The main elimination route of this compound is the oxidative metabolism via CYP3A4/2C8 and biliary excretion . Among the three major metabolites identified, hydroxy-dabrafenib appears to contribute to the pharmacological activity .
Transport and Distribution
This compound is almost completely absorbed after administration of the recommended dose of 150 mg twice daily . It shows a time-dependent increase in apparent clearance following multiple doses, which is likely due to induction of its own metabolism through cytochrome P450 (CYP) 3A4 .
Subcellular Localization
The distinctive subcellular localization of PCTAIRE CDKs in the cytoplasm and at the plasma membrane, often in association with cytoskeletal elements, suggests roles in vesicular transport, adhesion, and cell motility
Vorbereitungsmethoden
Die Synthese von Dabrafenib umfasst mehrere wichtige Schritte, darunter Sulfamidierung, Halogenierung, Thiazol-Cyclisierung, Acylierung und Pyrimidin-Cyclisierung . Der Prozess beginnt mit 3-(3-Amino-2-fluorphenyl)-3-oxa-propionat als Ausgangsmaterial. Diese Verbindung durchläuft eine Reihe von Reaktionen, um das Endprodukt this compound zu bilden . Industrielle Produktionsverfahren sind so konzipiert, dass sie prägnant und mild sind, was sie für die großtechnische Herstellung geeignet macht .
Analyse Chemischer Reaktionen
Dabrafenib durchläuft verschiedene chemische Reaktionen, die hauptsächlich den oxidativen Metabolismus über Cytochrom-P450-Enzyme CYP3A4 und CYP2C8 beinhalten . Die gebildeten Hauptmetaboliten umfassen Hydroxy-Dabrafenib, das zur pharmakologischen Aktivität der Verbindung beiträgt . Häufig verwendete Reagenzien in diesen Reaktionen sind Formamid und verschiedene Basen . Die Hauptprodukte dieser Reaktionen sind die Metaboliten, die in Galle und Urin ausgeschieden werden .
Wissenschaftliche Forschungsanwendungen
Dabrafenib hat eine breite Palette von Anwendungen in der wissenschaftlichen Forschung, insbesondere in den Bereichen Onkologie und Pharmakologie. Es wird verwendet, um die Auswirkungen von BRAF-Mutationen auf die Zellproliferation und das Tumorwachstum zu untersuchen . In Kombination mit Trametinib hat sich gezeigt, dass this compound die Ansprechraten und das progressionsfreie Überleben bei Patienten mit BRAF V600E-mutierten Krebserkrankungen verbessert . Darüber hinaus wird this compound in präklinischen Studien eingesetzt, um sein Potenzial bei der Behandlung anderer Krebsarten wie Darmkrebs und nicht-kleinzelligem Lungenkrebs zu untersuchen .
Wirkmechanismus
This compound ist ein kompetitiver und selektiver Inhibitor der BRAF-Kinase, der speziell die ATP-Tasche des Enzyms anvisiert . Es hat eine höhere Affinität zu mutierten Formen von BRAF, einschließlich BRAF V600E, BRAF V600K und BRAF V600D . Durch die Hemmung von BRAF stört this compound den MAPK-Signalweg, was zu einem Zellzyklusarrest und Apoptose in Krebszellen führt . Dieser Wirkmechanismus macht this compound effektiv bei der Behandlung von Krebserkrankungen, die durch BRAF-Mutationen getrieben werden .
Vergleich Mit ähnlichen Verbindungen
Dabrafenib wird oft mit anderen BRAF-Inhibitoren verglichen, wie z. B. Vemurafenib und Encorafenib . Während alle drei Verbindungen die BRAF-Kinase anvisieren, verfügt this compound über eine einzigartige chemische Struktur, die eine unterschiedliche Bindungsaffinität und Selektivität ermöglicht . Diese Einzigartigkeit trägt zu seinen unterschiedlichen pharmakokinetischen und pharmakodynamischen Profilen bei . Andere ähnliche Verbindungen umfassen Trametinib, das oft in Kombination mit this compound verwendet wird, um seine therapeutische Wirkung zu verstärken .
Eigenschaften
IUPAC Name |
N-[3-[5-(2-aminopyrimidin-4-yl)-2-tert-butyl-1,3-thiazol-4-yl]-2-fluorophenyl]-2,6-difluorobenzenesulfonamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C23H20F3N5O2S2/c1-23(2,3)21-30-18(19(34-21)16-10-11-28-22(27)29-16)12-6-4-9-15(17(12)26)31-35(32,33)20-13(24)7-5-8-14(20)25/h4-11,31H,1-3H3,(H2,27,28,29) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
BFSMGDJOXZAERB-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)(C)C1=NC(=C(S1)C2=NC(=NC=C2)N)C3=C(C(=CC=C3)NS(=O)(=O)C4=C(C=CC=C4F)F)F | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C23H20F3N5O2S2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID20152499 | |
| Record name | Dabrafenib | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID20152499 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
519.6 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
very slightly soluble at pH 1 | |
| Record name | Dabrafenib | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08912 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Mechanism of Action |
Dabrafenib is a competitive and selective BRAF inhibitor by binding to its ATP pocket.. Although dabrafenib can inhibit wild-type BRAF, it has a higher affinity for mutant forms of BRAF, including BRAF V600E, BRAF V600K, and BRAF V600D. BRAF is a serine/threonine protein kinase and is involved in activating the RAS/RAF/MEK/ERK or MAPK pathway, a pathway that is implicated in cell cycle progression, cell proliferation, and arresting apoptosis.Therefore, constitutive active mutation of BRAF such as BRAF V600E is frequently observed in many types of cancer, including melanoma, lung cancer, and colon cancer. | |
| Record name | Dabrafenib | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08912 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
1195765-45-7 | |
| Record name | Dabrafenib | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=1195765-45-7 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Dabrafenib [USAN:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=1195765457 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Dabrafenib | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08912 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Dabrafenib | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID20152499 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | N-[3-[5-(2-aminopyrimidin-4-yl)-2-tert-butyl-1,3-thiazol-4-yl]-2-fluorophenyl]-2,6-difluorobenzenesulfonamide | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.215.965 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | DABRAFENIB | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/QGP4HA4G1B | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Synthesis routes and methods I
Procedure details





Synthesis routes and methods II
Procedure details





Synthesis routes and methods III
Procedure details






Q1: What is the primary molecular target of Dabrafenib?
A1: this compound is a potent ATP-competitive inhibitor specifically targeting the V600 mutant B-Raf kinase (BRAF), primarily BRAF V600E and to a lesser extent BRAF V600K. [, , , ]
Q2: How does this compound's inhibition of BRAF affect downstream signaling?
A2: this compound binding to BRAF prevents the phosphorylation and activation of downstream MEK proteins (MEK1 and MEK2), effectively inhibiting the RAS/RAF/MEK/ERK (MAPK) signaling pathway. This pathway is crucial for cell division, differentiation, and various developmental processes. [, , ]
Q3: What is the molecular formula and weight of this compound?
A3: The provided research papers do not explicitly state the molecular formula and weight of this compound. Please refer to drug information resources like PubChem or Drugbank for this information.
Q4: Is there any spectroscopic data available for this compound in the research?
A4: The research papers do not provide detailed spectroscopic data for this compound.
Q5: How does light exposure affect this compound stability?
A5: this compound exhibits photosensitivity in both organic and aqueous solutions, leading to its degradation and the formation of a novel fluorescent derivative, dabrafenib_photo 2. []
Q6: What are the implications of this compound's photosensitivity?
A6: Researchers handling this compound should be aware of its photosensitivity and take precautions during storage and experiments to minimize light exposure. This includes using amber vials for storage and performing experiments under controlled lighting conditions. []
Q7: Does this compound exhibit any catalytic properties itself?
A7: this compound is not described as possessing intrinsic catalytic properties. It functions as an enzyme inhibitor, specifically targeting BRAF kinase.
Q8: Has computational chemistry been used to study this compound?
A8: The provided research papers do not extensively discuss computational chemistry studies specific to this compound.
Q9: What is known about the Structure-Activity Relationship (SAR) of this compound?
A9: While specific SAR studies are not detailed in the research, it's understood that modifications impacting this compound's interaction with the ATP-binding site of BRAF V600E/K will alter its potency and selectivity. [, ]
Q10: What is the recommended storage for this compound?
A10: Given its photosensitivity, this compound should be stored in amber vials to protect from light degradation. Specific temperature and storage conditions would be outlined in the manufacturer's information. []
Q11: Do the provided research papers discuss SHE regulations related to this compound?
A11: The research papers primarily focus on this compound's biological activity and clinical effects and do not delve into SHE regulations.
Q12: How is this compound metabolized in the body?
A12: this compound is primarily metabolized by CYP2C8 (56%-67%) and CYP3A4 (24%) enzymes in the liver. []
Q13: Are there any known drug-drug interactions (DDIs) with this compound?
A13: this compound is a potential inducer of CYP3A4 and can be a victim of both CYP3A4 and CYP2C8 inhibitors. Co-administration with strong inhibitors of these enzymes, like ketoconazole (CYP3A4) or gemfibrozil (CYP2C8), can significantly increase this compound exposure. [, ]
Q14: Does this compound affect the metabolism of other drugs?
A14: Yes, this compound has shown inhibition of several CYP enzymes in vitro, including CYP2C8, 2C9, 2C19, 3A4. Notably, it can decrease the AUC of S-warfarin, a CYP2C9 substrate, indicating a potential for interaction. [, ]
Q15: What is the clinical significance of this compound's interaction with CYP enzymes?
A15: Clinicians should be cautious when co-prescribing this compound with drugs metabolized by CYP2C8 and CYP3A4, as dose adjustments may be necessary to avoid adverse events or reduced efficacy. Monitoring for potential drug interactions is essential. [, , ]
Q16: Which human melanoma cell lines have been used to study this compound's efficacy in vitro?
A16: Several human melanoma cell lines, including A375, 397, 624.38, WM3918, WM9838BR, WM983B, and DB-1, have been used in various studies to assess this compound's impact on cell signaling, proliferation, and response to treatment. [, , ]
Q17: What in vivo models have been used to study this compound?
A17: Mouse xenograft models implanted with human melanoma cells have been used to assess this compound's efficacy in vivo, allowing for tumor growth monitoring and assessment of therapeutic response. []
Q18: What are the key findings from clinical trials evaluating this compound?
A18: Clinical trials have demonstrated that this compound, as monotherapy or combined with Trametinib (a MEK inhibitor), significantly improves progression-free survival and overall survival in patients with BRAF V600E/K-mutated melanoma. [, , , , ]
Q19: What are the mechanisms of resistance to this compound?
A19: One of the primary resistance mechanisms to this compound involves the reactivation of the MAPK pathway, often through the development of new mutations in NRAS or KRAS, or through upregulation of growth factor receptors like EGFR. [, , ]
Q20: Is there cross-resistance between this compound and other BRAF inhibitors?
A20: Cross-resistance between this compound and other BRAF inhibitors, such as Vemurafenib, can occur. This is often due to shared resistance mechanisms involving reactivation of the MAPK pathway or other signaling pathways that bypass BRAF inhibition. []
Q21: What are the common cutaneous side effects observed with this compound?
A21: Common cutaneous side effects include papillomas, palmoplantar hyperkeratosis, alopecia, and seborrheic dermatitis-like eruption. In some cases, development of keratoacanthoma-like squamous cell carcinoma has been reported. []
Q22: Are there any specific drug delivery strategies being explored for this compound?
A22: The research papers provided do not delve into specific drug delivery strategies being investigated for this compound.
Q23: What is the primary biomarker for selecting patients for this compound treatment?
A23: The presence of BRAF V600E or V600K mutations in tumor tissue is the primary biomarker for identifying patients who are eligible for treatment with this compound. [, , , ]
Q24: Are there any biomarkers being investigated to monitor response to this compound treatment?
A24: Research suggests that circulating levels of the PTTG1 protein might serve as a potential biomarker for monitoring patient response to targeted therapy, including this compound. []
Q25: What analytical techniques are commonly used to quantify this compound and its metabolites?
A25: Liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) is commonly employed for quantifying this compound and its metabolites in biological samples. [, ]
Q26: Do the provided research papers discuss the environmental impact of this compound?
A26: The environmental impact and degradation of this compound are not discussed in the provided research papers.
Q27: Are there any studies on the dissolution and solubility of this compound?
A27: The provided research papers do not specifically address the dissolution and solubility characteristics of this compound.
Q28: What are the key parameters assessed during the validation of analytical methods for this compound?
A28: While not explicitly detailed, validation of analytical methods like LC-MS/MS for this compound quantification would involve assessing accuracy, precision, specificity, linearity, range, limit of detection, limit of quantification, and robustness. [, , ]
Q29: Do the research papers discuss specific quality control measures for this compound?
A29: The research papers focus on scientific aspects and do not delve into detailed quality control measures employed during this compound development and manufacturing.
Q30: Does this compound have any known impact on the immune system?
A30: this compound, while not directly targeting the immune system, has been shown to impact the tumor microenvironment. Research suggests it can modulate the differentiation and function of myeloid-derived suppressor cells (MDSCs), which play a role in immune suppression within tumors. [, ]
Q31: Are there any known interactions between this compound and drug transporters?
A31: Specific information regarding interactions between this compound and drug transporters is not provided in the research papers.
Q32: Does this compound induce or inhibit drug-metabolizing enzymes?
A32: this compound has been shown to both inhibit and induce drug-metabolizing enzymes. It inhibits various CYP enzymes (CYP2C8, 2C9, 2C19, 3A4), potentially affecting the metabolism of co-administered drugs. Conversely, it can also induce CYP3A4, leading to potential alterations in its own metabolism and that of other CYP3A4 substrates. [, , ]
Q33: Is there information on this compound's biocompatibility and biodegradability?
A33: The provided research papers do not focus on this compound's biocompatibility or biodegradability aspects.
Q34: What are alternative treatment options for BRAF V600E/K-mutated melanoma?
A34: Other BRAF inhibitors like Vemurafenib, as well as MEK inhibitors like Trametinib and Cobimetinib, are alternative treatment options for BRAF V600E/K-mutated melanoma. Immunotherapy options, including immune checkpoint inhibitors like Nivolumab and Ipilimumab, are also used in the treatment of advanced melanoma. [, , , ]
Q35: Do the research papers mention strategies for recycling or managing this compound waste?
A35: The research papers do not address strategies for recycling or managing this compound waste.
Q36: What research infrastructure and resources are important for studying this compound?
A36: Important resources include access to well-characterized human melanoma cell lines, in vivo models like mouse xenografts, high-throughput screening platforms for evaluating drug efficacy and potential resistance mechanisms, and advanced analytical techniques like LC-MS/MS for quantifying drug concentrations and monitoring metabolic activity.
Q37: When was this compound approved for the treatment of melanoma?
A37: this compound received FDA approval for the treatment of metastatic melanoma harboring the BRAF V600E mutation in 2013. [, ]
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.

![4-tert-butyl-N-[6-[2-[6-[(4-tert-butylphenyl)sulfonylamino]-5-(2-methoxyphenoxy)-2-pyrimidin-2-ylpyrimidin-4-yl]oxyethoxy]-5-(2-methoxyphenoxy)-2-pyrimidin-2-ylpyrimidin-4-yl]benzenesulfonamide](/img/structure/B601009.png)
