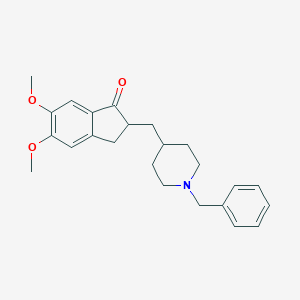
Donepezil
Descripción general
Descripción
Donepezilo es un medicamento utilizado principalmente para tratar la demencia asociada con la enfermedad de Alzheimer. Es un inhibidor reversible de la acetilcolinesterasa de acción central, lo que significa que funciona inhibiendo la enzima acetilcolinesterasa, lo que aumenta la concentración de acetilcolina en el cerebro. Este aumento en la acetilcolina ayuda a mejorar la función cognitiva y la memoria en pacientes con enfermedad de Alzheimer .
Métodos De Preparación
Rutas sintéticas y condiciones de reacción: La síntesis de donepezilo generalmente involucra la condensación aldólica de bencilpiperidina-carboxaldehído con dimetoxiindanona, utilizando la reacción de Wittig. Esto va seguido de un paso de deshidratación posterior y reducción catalítica del doble enlace exocíclico, produciendo el producto deseado .
Métodos de producción industrial: La producción industrial de donepezilo implica rutas sintéticas similares, pero a mayor escala. El proceso está optimizado para la eficiencia, la rentabilidad y la escalabilidad. También se están explorando estrategias ecológicas para reducir el impacto ambiental del proceso de producción .
Análisis De Reacciones Químicas
Tipos de reacciones: Donepezilo sufre varias reacciones químicas, incluida la oxidación, la reducción y la sustitución.
Reactivos y condiciones comunes:
Oxidación: Donepezilo se puede oxidar utilizando oxidantes suaves como la cloramina-T en un medio ácido.
Reducción: La reducción catalítica se utiliza en el proceso de síntesis para reducir el doble enlace exocíclico.
Productos principales: Los principales productos formados a partir de estas reacciones incluyen varios análogos y derivados de donepezilo, que se exploran por sus posibles beneficios terapéuticos .
Aplicaciones Científicas De Investigación
Donepezilo tiene una amplia gama de aplicaciones de investigación científica:
Medicina: Donepezilo se utiliza ampliamente en el tratamiento de la enfermedad de Alzheimer y otros tipos de demencia.
Industria: La industria farmacéutica utiliza donepezilo en el desarrollo de nuevos fármacos y agentes terapéuticos.
Mecanismo De Acción
Donepezilo inhibe selectivamente y de forma reversible la enzima acetilcolinesterasa, que normalmente descompone la acetilcolina. Al inhibir esta enzima, donepezilo aumenta la concentración de acetilcolina en el cerebro, mejorando la transmisión colinérgica. Esto ayuda a aliviar los síntomas de la demencia de Alzheimer al mejorar la función cognitiva y la memoria .
Compuestos similares:
Rivastigmina: Otro inhibidor de la acetilcolinesterasa utilizado para tratar la demencia. A diferencia de donepezilo, también inhibe la butirilcolinesterasa.
Galantamina: Un inhibidor de la acetilcolinesterasa que también modula los receptores nicotínicos.
Singularidad de Donepezilo: Donepezilo es único en su inhibición selectiva y reversible de la acetilcolinesterasa, lo que lo hace altamente efectivo para aumentar los niveles de acetilcolina en el cerebro. Su larga vida media y su capacidad para cruzar la barrera hematoencefálica contribuyen a su eficacia en el manejo de los síntomas de la enfermedad de Alzheimer .
Comparación Con Compuestos Similares
Rivastigmine: Another acetylcholinesterase inhibitor used to treat dementia. Unlike donepezil, it also inhibits butyrylcholinesterase.
Galantamine: An acetylcholinesterase inhibitor that also modulates nicotinic receptors.
Uniqueness of this compound: this compound is unique in its selective and reversible inhibition of acetylcholinesterase, which makes it highly effective in increasing acetylcholine levels in the brain. Its long half-life and ability to cross the blood-brain barrier contribute to its effectiveness in managing Alzheimer’s disease symptoms .
Propiedades
IUPAC Name |
2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimethoxy-2,3-dihydroinden-1-one | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
ADEBPBSSDYVVLD-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
COC1=C(C=C2C(=C1)CC(C2=O)CC3CCN(CC3)CC4=CC=CC=C4)OC | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C24H29NO3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
120011-70-3 | |
| Record name | Donepezil [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0120014064 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID8048317 | |
| Record name | Donepezil | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8048317 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
379.5 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Donepezil | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0005041 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
31mg/mL | |
| Record name | Donepezil | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00843 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Mechanism of Action |
The commonly accepted cholinergic hypothesis proposes that a portion of the cognitive and behavioral decline associated with Alzheimer's are the result of decreased cholinergic transmission in the central nervous system. Donepezil selectively and reversibly inhibits the acetylcholinesterase enzyme, which normally breaks down acetylcholine. The main pharmacological actions of this drug are believed to occur as the result of this enzyme inhibition, enhancing cholinergic transmission, which relieves the symptoms of Alzheimer's dementia. In addition to the above, other mechanisms of action of donepezil are possible, including the opposition of glutamate-induced excitatory transmission via downregulation of NMDA receptors and the regulation of amyloid proteins, which have demonstrated significant effects on the disease process of Alzheimer's. Other possible targets for donepezil may also include the inhibition various inflammatory signaling pathways, exerting neuroprotective effects., Donepezil hydrochloride, a piperidine derivative, is a centrally active, reversible inhibitor of acetylcholinesterase. The drug is structurally unrelated to other anticholinesterase agents (eg, tacrine, physostigmine)., The precise mechanism(s) of action of donepezil in patients with dementia of the Alzheimer's type (Alzheimer's disease) has not been fully elucidated. The drug is an anticholinesterase agent that binds reversibly with and inactivates cholinesterases (eg, acetylcholinesterase), thus inhibiting hydrolysis of acetylcholine. As a result, the concentration of acetylcholine increases at cholinergic synapses. In vitro data and data in animals indicate that the anticholinesterase activity of donepezil is relatively specific for acetylcholinesterase in the brain compared with butyrylcholinesterase inhibition in peripheral tissues., A deficiency of acetylcholine caused by selective loss of cholinergic neurons in the cerebral cortex, nucleus basalis, and hippocampus is recognized as one of the early pathophysiologic features of Alzheimer's disease associated with memory loss and cognitive deficits. Because the resultant cortical deficiency of this neurotransmitter is believed to account for some of the clinical manifestations of mild to moderate dementia, enhancement of cholinergic function with an anticholinesterase agent, such as tacrine or donepezil, is one of the pharmacologic approaches to treatment. Because widespread degeneration of multiple central neuronal systems eventually occurs in patients with Alzheimer's disease, potentially beneficial effects of anticholinesterase agents theoretically would diminish as the disease process advances and fewer cholinergic neurons remain functioning., Current theories on the pathogenesis of the cognitive signs and symptoms of Alzheimer's Disease attribute some of them to a deficiency of cholinergic neurotransmission. Donepezil hydrochloride is postulated to exert its therapeutic effect by enhancing cholinergic function. This is accomplished by increasing the concentration of acetylcholine through reversible inhibition of its hydrolysis by acetylcholinesterase. There is no evidence that donepezil alters the course of the underlying dementing process., For more Mechanism of Action (Complete) data for Donepezil (6 total), please visit the HSDB record page. | |
| Record name | Donepezil | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00843 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Donepezil | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7743 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
CAS No. |
120014-06-4 | |
| Record name | Donepezil | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=120014-06-4 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Donepezil [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0120014064 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Donepezil | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00843 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Donepezil | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8048317 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | DONEPEZIL | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/8SSC91326P | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Donepezil | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7743 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Donepezil | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0005041 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
223-227 | |
| Record name | Donepezil | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00843 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Synthesis routes and methods I
Procedure details




Synthesis routes and methods II
Procedure details





Synthesis routes and methods III
Procedure details





Synthesis routes and methods IV
Procedure details








Synthesis routes and methods V
Procedure details









Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.
![2-[(1S)-2-[(2-methylpropan-2-yl)oxy]-2-oxo-1-(phenylmethoxycarbonylamino)ethyl]pent-4-enoic acid](/img/structure/B133171.png)