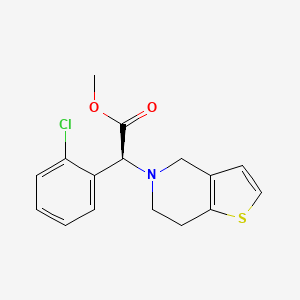
Clopidogrel
Descripción general
Descripción
Clopidogrel es un medicamento antiplaquetario que se utiliza principalmente para reducir el riesgo de enfermedades cardíacas y accidentes cerebrovasculares en individuos con alto riesgo. A menudo se prescribe en combinación con aspirina para pacientes que han experimentado ataques cardíacos o se han sometido a la colocación de un stent de arteria coronaria . This compound pertenece a la clase de agentes antiplaquetarios de tienopiridina y funciona inhibiendo la agregación plaquetaria .
Mecanismo De Acción
Clopidogrel es un profármaco que requiere biotransformación in vivo a un metabolito activo de tiol. Este metabolito activo se une irreversiblemente al componente P2Y12 de los receptores de difosfato de adenosina en la superficie de las plaquetas . Esta unión evita que el difosfato de adenosina active el complejo receptor de glucoproteína GPIIb/IIIa, reduciendo así la agregación plaquetaria y previniendo la formación de coágulos .
Compuestos similares:
Ticlopidina: Otro agente antiplaquetario de tienopiridina, pero con una mayor incidencia de efectos adversos.
Prasugrel: Una tienopiridina más nueva con una aparición más rápida de la acción y una mayor potencia en comparación con this compound.
Ticagrelor: Un agente antiplaquetario no tienopiridínico que proporciona una inhibición reversible del receptor P2Y12.
Singularidad de this compound: this compound es único debido a su unión irreversible al receptor P2Y12, proporcionando efectos antiplaquetarios duraderos. También se utiliza ampliamente en la práctica clínica debido a su eficacia probada y perfil de seguridad .
Análisis Bioquímico
Biochemical Properties
Clopidogrel’s biochemical target is the purinergic receptor P2Y12 . In platelets, the P2Y12 receptor is coupled to the inhibitory G-protein Gi . The inhibition of platelet response to ADP is observed within 12 hours after this compound intake .
Cellular Effects
This compound has significant effects on platelets. It inhibits the function of the P2Y12 receptor, thereby preventing spontaneous platelet aggregation after percutaneous transluminal coronary angioplasty .
Molecular Mechanism
This compound is a prodrug that requires biotransformation into its active thiol metabolite by the cytochrome P450 enzymes . The active metabolite then inhibits the P2Y12 receptor on platelets, preventing platelet aggregation .
Temporal Effects in Laboratory Settings
In laboratory settings, aggregometry and VASP phosphorylation revealed a loss of platelet response to ADP within 12 hours after this compound intake . The phosphorylation status of VASP correlated with the inhibition of platelet aggregation .
Metabolic Pathways
This compound is metabolized by the cytochrome P450 enzymes, primarily CYP2C19, into its active form . The active metabolite then inhibits the P2Y12 receptor, affecting platelet aggregation .
Métodos De Preparación
Rutas sintéticas y condiciones de reacción: Clopidogrel se puede sintetizar mediante varios métodos. Un enfoque común implica la racemización de R(-) this compound utilizando una base anhidra en polvo en uno o más solventes . Otro método incluye la precipitación de this compound bisulfato a partir de una mezcla de disolventes de metanol y acetona en presencia de ácido sulfúrico a una temperatura de 25-40°C . Además, this compound se puede preparar haciendo reaccionar L-2-clorofenilglicina con metanol para generar éster metílico de L-2-clorofenilglicina, seguido de una serie de reacciones para obtener this compound .
Métodos de producción industrial: La producción industrial de this compound a menudo implica la resolución de this compound racèmico para obtener el enantiómero activo S(+). Este proceso incluye el uso de reactivos y condiciones específicas para asegurar un alto rendimiento y pureza .
Análisis De Reacciones Químicas
Tipos de reacciones: Clopidogrel se somete a varios tipos de reacciones químicas, incluidas la oxidación, la reducción y la sustitución.
Reactivos y condiciones comunes:
Oxidación: this compound se puede oxidar utilizando reactivos como peróxido de hidrógeno o permanganato de potasio en condiciones controladas.
Reducción: Las reacciones de reducción pueden involucrar el uso de agentes reductores como borohidruro de sodio o hidruro de aluminio y litio.
Sustitución: Las reacciones de sustitución a menudo requieren nucleófilos como haluros o aminas en presencia de catalizadores adecuados.
Productos principales: Los productos principales formados a partir de estas reacciones dependen de los reactivos y las condiciones específicas utilizados. Por ejemplo, la oxidación de this compound puede conducir a la formación de varios metabolitos oxidados, mientras que las reacciones de sustitución pueden producir diferentes derivados sustituidos de this compound.
Aplicaciones Científicas De Investigación
Clopidogrel tiene una amplia gama de aplicaciones de investigación científica:
Química: this compound se utiliza como compuesto modelo en estudios relacionados con agentes antiplaquetarios y sus mecanismos de acción.
Biología: La investigación sobre this compound se centra en sus efectos sobre la función plaquetaria y su papel en la prevención de eventos trombóticos.
Medicina: this compound se estudia ampliamente por sus aplicaciones clínicas en la prevención de ataques cardíacos, accidentes cerebrovasculares y otros eventos cardiovasculares.
Comparación Con Compuestos Similares
Ticlopidine: Another thienopyridine antiplatelet agent, but with a higher incidence of adverse effects.
Prasugrel: A newer thienopyridine with a more rapid onset of action and greater potency compared to clopidogrel.
Ticagrelor: A non-thienopyridine antiplatelet agent that provides reversible inhibition of the P2Y12 receptor.
Uniqueness of this compound: this compound is unique due to its irreversible binding to the P2Y12 receptor, providing long-lasting antiplatelet effects. It is also widely used in clinical practice due to its proven efficacy and safety profile .
Propiedades
IUPAC Name |
methyl (2S)-2-(2-chlorophenyl)-2-(6,7-dihydro-4H-thieno[3,2-c]pyridin-5-yl)acetate | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C16H16ClNO2S/c1-20-16(19)15(12-4-2-3-5-13(12)17)18-8-6-14-11(10-18)7-9-21-14/h2-5,7,9,15H,6,8,10H2,1H3/t15-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
GKTWGGQPFAXNFI-HNNXBMFYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
COC(=O)C(C1=CC=CC=C1Cl)N2CCC3=C(C2)C=CS3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
COC(=O)[C@H](C1=CC=CC=C1Cl)N2CCC3=C(C2)C=CS3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C16H16ClNO2S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID6022848 | |
| Record name | Clopidogrel | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID6022848 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
321.8 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Clopidogrel | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0005011 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
15.1 [ug/mL] (The mean of the results at pH 7.4) | |
| Record name | SID49666423 | |
| Source | Burnham Center for Chemical Genomics | |
| URL | https://pubchem.ncbi.nlm.nih.gov/bioassay/1996#section=Data-Table | |
| Description | Aqueous solubility in buffer at pH 7.4 | |
Mechanism of Action |
Clopidogrel is metabolized to its active form by carboxylesterase-1. The active form is a platelet inhibitor that irreversibly binds to P2Y12 ADP receptors on platelets. This binding prevents ADP binding to P2Y12 receptors, activation of the glycoprotein GPIIb/IIIa complex, and platelet aggregation., Clopidogrel must be metabolized by CYP450 enzymes to produce the active metabolite that inhibits platelet aggregation. The active metabolite of clopidogrel selectively inhibits the binding of adenosine diphosphate (ADP) to its platelet P2Y12 receptor and the subsequent ADP-mediated activation of the glycoprotein GPIIb/IIIa complex, thereby inhibiting platelet aggregation. This action is irreversible. Consequently, platelets exposed to clopidogrel's active metabolite are affected for the remainder of their lifespan (about 7 to 10 days). Platelet aggregation induced by agonists other than ADP is also inhibited by blocking the amplification of platelet activation by released ADP., The P2Y12 receptor plays a crucial role in the regulation of platelet activation by several agonists, which is irreversibly antagonized by the active metabolite of clopidogrel, a widely used anti-thrombotic drug. In this study, we investigated whether reduction of platelet reactivity leads to reduced inflammatory responses using a rat model of erosive arthritis. We evaluated the effect of clopidogrel on inflammation in Lewis rats in a peptidoglycan polysaccharide (PG-PS)-induced arthritis model with four groups of rats: 1) untreated, 2) clopidogrel-treated, 3) PG-PS-induced, and 4) PG-PS-induced and clopidogrel-treated. There were significant differences between the PG-PS+clopidogrel group when compared to the PG-PS group including: increased joint diameter and clinical manifestations of inflammation, elevated plasma levels of pro-inflammatory cytokines (IL-1 beta, interferon (IFN) gamma, and IL-6), an elevated neutrophil blood count and an increased circulating platelet count. Plasma levels of IL-10 were significantly lower in the PG-PS+clopidogrel group compared to the PG-PS group. Plasma levels of platelet factor 4 (PF4) were elevated in both the PG-PS and the PG-PS+clopidogrel groups, however PF4 levels showed no difference upon clopidogrel treatment, suggesting that the pro- inflammatory effect of clopidogrel may be due to its action on cells other than platelets. Histology indicated an increase in leukocyte infiltration at the inflammatory area of the joint, increased pannus formation, blood vessel proliferation, subsynovial fibrosis and cartilage erosion upon treatment with clopidogrel in PG-PS-induced arthritis animals. In summary, animals treated with clopidogrel showed a pro-inflammatory effect in the PG-PS-induced arthritis animal model, which might not be mediated by platelets. | |
| Record name | Clopidogrel | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00758 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | CLOPIDOGREL | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7430 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Colorless oil | |
CAS No. |
113665-84-2 | |
| Record name | Clopidogrel | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=113665-84-2 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Clopidogrel [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0113665842 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Clopidogrel | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00758 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Clopidogrel | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID6022848 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Thieno[3,2-c]pyridine-5(4H)-acetic acid, α-(2-chlorophenyl)-6,7-dihydro-, methyl ester, (αS) | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.127.841 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | CLOPIDOGREL | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/A74586SNO7 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | CLOPIDOGREL | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7430 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Clopidogrel | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0005011 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods I
Procedure details





Synthesis routes and methods II
Procedure details





Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: How does Clopidogrel interact with its target and what are the downstream effects?
A1: this compound is a prodrug that requires metabolic activation to exert its antiplatelet effect. Its active metabolite irreversibly binds to the platelet adenosine diphosphate (ADP) receptor P2Y12. [, , , , ] This binding prevents ADP-induced platelet activation and aggregation, crucial steps in thrombus formation. [, , , ] this compound's action ultimately reduces the risk of atherothrombotic events like stroke, myocardial infarction, and cardiovascular death. [, , , , , , , , ]
Q2: Why is P2Y12 inhibition important for antiplatelet therapy?
A2: The P2Y12 receptor on platelets plays a crucial role in amplifying platelet activation initiated by various agonists, including ADP. [, , , , ] Inhibiting P2Y12 effectively blocks this amplification, leading to a more sustained reduction in platelet aggregation and thrombus formation.
Q3: How does this compound compare to Aspirin in terms of platelet inhibition?
A3: While both this compound and Aspirin are antiplatelet agents, they target different pathways. Aspirin inhibits the cyclooxygenase enzyme, thereby blocking Thromboxane A2 synthesis, a potent platelet activator. This compound, on the other hand, blocks the P2Y12 receptor, preventing ADP-induced platelet activation. [, , , ] Combining both agents can result in synergistic platelet inhibition, especially in acute coronary syndromes. [, , , , , ]
Q4: What is the molecular formula and weight of this compound?
A4: The molecular formula of this compound is C16H16ClNO2S, and its molecular weight is 321.82 g/mol.
Q5: How is this compound metabolized, and how does this impact its effectiveness?
A5: this compound is a prodrug, meaning it needs to be metabolized in the liver to become active. This process involves several enzymes, particularly CYP2C19. [, , , , , , ] Genetic variations in CYP2C19 can significantly impact this compound metabolism and, consequently, its effectiveness. [, , , , , , ]
Q6: What is this compound resistance, and how does it develop?
A6: this compound resistance refers to an inadequate platelet inhibition despite taking this compound. [, , , ] It can result from factors such as genetic polymorphisms in CYP2C19, drug interactions (e.g., with proton pump inhibitors), diabetes, obesity, and individual variability in platelet reactivity. [, , , , , , , ]
Q7: What are the main clinical uses of this compound?
A8: this compound is primarily used to prevent atherothrombotic events in patients with acute coronary syndromes (including unstable angina and myocardial infarction), those who have undergone percutaneous coronary interventions (PCI), and individuals with a history of stroke or peripheral artery disease. [, , , , , , , , ]
Q8: What are the advantages of using this compound as a loading dose before PCI?
A9: Administering a loading dose of this compound before PCI helps achieve rapid and effective platelet inhibition, reducing the risk of stent thrombosis and other ischemic complications. [, , , ] Clinical trials have demonstrated a greater reduction in ischemic events when this compound is given as a loading dose compared to placebo or lower doses. [, , ]
Q9: What are the implications of the interaction between this compound and Proton Pump Inhibitors (PPIs)?
A10: PPIs, commonly prescribed to prevent gastrointestinal bleeding in patients on dual antiplatelet therapy, can inhibit the CYP2C19 enzyme responsible for this compound activation. [, , , ] This interaction may reduce this compound's effectiveness and potentially increase the risk of adverse cardiovascular events in some patients. [, , , ]
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.
![(E)-[6]-Dehydroparadol](/img/structure/B1663509.png)
![methyl (4S)-2,6-dimethyl-5-nitro-4-[2-(trifluoromethyl)phenyl]-1,4-dihydropyridine-3-carboxylate](/img/structure/B1663520.png)
![(5E)-5-[[3-(trifluoromethyl)phenyl]methylidene]-1,3-thiazolidine-2,4-dione](/img/structure/B1663525.png)