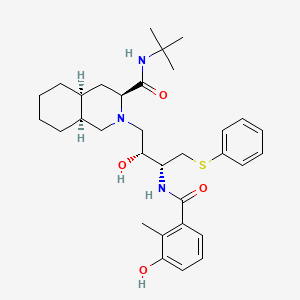
Nelfinavir
Descripción general
Descripción
Nelfinavir es un medicamento antirretroviral utilizado en el tratamiento del VIH/SIDA. This compound es un inhibidor de la proteasa del virus de la inmunodeficiencia humana tipo 1 (VIH-1) biodisponible por vía oral y fue patentado en 1992 y aprobado para uso médico en 1997 .
Aplicaciones Científicas De Investigación
Nelfinavir tiene una amplia gama de aplicaciones de investigación científica. Se ha utilizado en el tratamiento de la infección por VIH tanto en adultos como en niños . Además, this compound se ha investigado por su posible uso en el tratamiento de la COVID-19. Se están llevando a cabo ensayos clínicos para evaluar su eficacia en casos de COVID-19 leves a moderados . This compound también ha mostrado promesas como agente anticancerígeno, inhibiendo la proliferación celular en múltiples cánceres y regulando el ciclo celular . Además, se ha descubierto que this compound inhibe la producción de factores de virulencia en diversas bacterias, incluida la Streptococcus pyogenes .
Mecanismo De Acción
Nelfinavir ejerce sus efectos inhibiendo la enzima proteasa viral del VIH, lo que evita la escisión de la poliproteína gag-pol, dando como resultado partículas virales inmaduras no infecciosas . La inhibición de la enzima proteasa es esencial para el ciclo de vida del VIH, ya que evita el ensamblaje de proteínas virales inmaduras en viriones maduros e infecciosos . This compound se une al sitio activo de la proteasa e inhibe su actividad, lo que lleva a la formación de partículas virales inmaduras no infecciosas .
Análisis Bioquímico
Biochemical Properties
Nelfinavir inhibits the HIV viral proteinase enzyme which prevents cleavage of the gag-pol polyprotein, resulting in noninfectious, immature viral particles . The inhibition of the HIV-1 protease enzyme is a key interaction of this compound in biochemical reactions .
Cellular Effects
This compound’s primary cellular effect is the prevention of the formation of infectious HIV-1 particles . By inhibiting the HIV-1 protease enzyme, this compound prevents the cleavage of the gag-pol polyprotein, which is necessary for the assembly of mature, infectious HIV-1 particles .
Molecular Mechanism
This compound binds to the protease active site and inhibits the activity of the enzyme . This inhibition prevents cleavage of the viral polyproteins resulting in the formation of immature non-infectious viral particles .
Temporal Effects in Laboratory Settings
In a study analyzing the pharmacokinetic profile of this compound, it was found that there is a high variability between individuals in this compound plasma concentrations . The mean average drug plasma concentration was 2.22 ± 1.25 mg/L and the mean AUC during the dosing interval was 17.7 ± 10.0 mg•h/L .
Dosage Effects in Animal Models
While specific dosage effects in animal models are not mentioned in the available literature, it is known that this compound is used in combination with other antiviral drugs in the treatment of HIV in both adults and children .
Metabolic Pathways
This compound is metabolized by multiple cytochrome P-450 enzymes including CYP3A and CYP2C19 . One major and several minor oxidative metabolites were found in plasma .
Transport and Distribution
Unchanged this compound comprised 82-86% of the total plasma radioactivity after a single oral 750 mg dose of 14C-Nelfinavir . This suggests that this compound is primarily present in its original form in the plasma, indicating its stability during transport and distribution within the body .
Subcellular Localization
The specific subcellular localization of this compound is not mentioned in the available literature. Given its role as a protease inhibitor, it is likely that this compound interacts with the HIV-1 protease enzyme in the cytoplasm of the cell, where the assembly of new viral particles takes place .
Métodos De Preparación
Nelfinavir puede sintetizarse a través de varios métodos. Un enfoque implica el desarrollo de nanocristales de Mesilato de this compound para superar los inconvenientes asociados con el fármaco, como la baja solubilidad y la biodisponibilidad oral . Los nanocristales se preparan utilizando una técnica combinada y un método de ultrasonido con alcohol polivinílico y poloxámero 407 como estabilizadores . Las características de estado sólido de los nanocristales optimizados se estudian mediante difracción de rayos X, espectroscopia infrarroja de transformada de Fourier, calorimetría de barrido diferencial y microscopía electrónica de barrido .
Análisis De Reacciones Químicas
Nelfinavir sufre varias reacciones químicas, incluida la oxidación, reducción y sustitución. Es un inhibidor de la proteasa con actividad contra el virus de la inmunodeficiencia humana tipo 1 (VIH-1) . Los inhibidores de la proteasa bloquean la parte del VIH llamada proteasa, que es una enzima necesaria para la escisión proteolítica de los precursores polipeptídicos virales en las proteínas funcionales individuales que se encuentran en el VIH-1 infeccioso . This compound se une al sitio activo de la proteasa e inhibe la actividad de la enzima, previniendo la escisión de las poliproteínas virales y provocando la formación de partículas virales inmaduras no infecciosas .
Comparación Con Compuestos Similares
Nelfinavir es único entre los inhibidores de la proteasa debido a su estructura y mecanismo de acción específicos. Compuestos similares incluyen otros inhibidores de la proteasa como ritonavir, lopinavir e indinavir . Estos compuestos también inhiben la enzima proteasa del VIH, pero pueden diferir en sus propiedades farmacocinéticas, efectos secundarios y eficacia . Se ha descubierto que this compound tiene una parte única de cis-decahidroisoquinolina-2-carboxamida, que puede proporcionar la base estructural para su mayor eficacia contra el cáncer en comparación con otros inhibidores de la proteasa del VIH .
Propiedades
IUPAC Name |
(3S,4aS,8aS)-N-tert-butyl-2-[(2R,3R)-2-hydroxy-3-[(3-hydroxy-2-methylbenzoyl)amino]-4-phenylsulfanylbutyl]-3,4,4a,5,6,7,8,8a-octahydro-1H-isoquinoline-3-carboxamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C32H45N3O4S/c1-21-25(15-10-16-28(21)36)30(38)33-26(20-40-24-13-6-5-7-14-24)29(37)19-35-18-23-12-9-8-11-22(23)17-27(35)31(39)34-32(2,3)4/h5-7,10,13-16,22-23,26-27,29,36-37H,8-9,11-12,17-20H2,1-4H3,(H,33,38)(H,34,39)/t22-,23+,26-,27-,29+/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
QAGYKUNXZHXKMR-HKWSIXNMSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1=C(C=CC=C1O)C(=O)NC(CSC2=CC=CC=C2)C(CN3CC4CCCCC4CC3C(=O)NC(C)(C)C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC1=C(C=CC=C1O)C(=O)N[C@@H](CSC2=CC=CC=C2)[C@@H](CN3C[C@H]4CCCC[C@H]4C[C@H]3C(=O)NC(C)(C)C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C32H45N3O4S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
159989-65-8 (monomethane sulfonate (salt)) | |
| Record name | Nelfinavir [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0159989647 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID5035080 | |
| Record name | Nelfinavir | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5035080 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
567.8 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Nelfinavir | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014365 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Slightly soluble, 1.91e-03 g/L | |
| Record name | Nelfinavir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00220 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Nelfinavir | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014365 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
HIV viral protease is an important enzyme for HIV maturation and pathogenicity since HIV produces its structural and key proteins in the form of a polyprotein that needs to be cleaved by a protease. HIV protease is synthesized as part of the Gag-pol polyprotein, where Gag encodes for the capsid and matrix protein to form the outer protein shell, and Pol encodes for the reverse transcriptase and integrase protein to synthesize and incorporate its genome into host cells. The Gag-pol polyprotein undergoes proteolytic cleavage by HIV protease to produce 66 molecular species which will assume conformational changes to become fully active. Inhibition of protease, therefore, prevents HIV virion from fully maturing and becoming infective. Nelfinavir is a competitive inhibitor of the HIV protease by reversibly binding to the active site of the enzyme, preventing it from interacting with its substrate to produce mature and infectious viral particles. | |
| Record name | Nelfinavir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00220 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
159989-64-7 | |
| Record name | Nelfinavir | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=159989-64-7 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Nelfinavir [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0159989647 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Nelfinavir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00220 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | nelfinavir | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=747167 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Nelfinavir | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5035080 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | NELFINAVIR | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/HO3OGH5D7I | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Nelfinavir | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014365 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
349.84 °C | |
| Record name | Nelfinavir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00220 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Nelfinavir | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014365 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary mechanism of action of Nelfinavir?
A1: this compound was initially developed as a specific inhibitor of human immunodeficiency virus (HIV) protease. [] It exerts its antiviral activity by binding to the active site of the HIV-1 protease, thereby preventing the cleavage of viral polyproteins and inhibiting viral maturation. []
Q2: How does this compound's activity extend to anti-cancer effects?
A2: Beyond its antiviral activity, this compound exhibits pleiotropic effects in various cancer cells. [, ] One key mechanism involves the induction of endoplasmic reticulum (ER) stress, leading to the unfolded protein response (UPR) and ultimately apoptosis (programmed cell death). [, , ] Additionally, this compound inhibits the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway, a key regulator of cell growth and survival, further contributing to its anti-cancer properties. [, ]
Q3: this compound has been shown to affect lipid metabolism in cancer cells. Can you elaborate?
A3: this compound inhibits the activity of Site-2 protease (S2P), an enzyme involved in the regulated intramembrane proteolysis (RIP) of sterol regulatory element binding protein-1 (SREBP-1) and activating transcription factor 6 (ATF6). [] This inhibition disrupts lipid homeostasis, contributing to ER stress and promoting cell death.
Q4: Can you explain the role of this compound in modulating the unfolded protein response (UPR) and its impact on cancer cells?
A4: this compound activates the UPR in myeloma cells, leading to the upregulation of UPR-related proteins. [] This activation can overcome proteasome inhibitor resistance often observed in multiple myeloma. [] The exact mechanisms underlying this effect are complex but involve modulation of key UPR sensors and downstream effectors.
Q5: How does this compound impact the tumor microenvironment?
A5: this compound downregulates hypoxia-inducible factor 1-alpha (HIF-1α) and vascular endothelial growth factor (VEGF) expression, leading to decreased angiogenesis (formation of new blood vessels). [] This, in turn, increases tumor oxygenation, potentially enhancing the efficacy of radiotherapy. []
Q6: What is the role of oxidative stress in this compound's anticancer activity?
A6: this compound has been shown to increase reactive oxygen species (ROS) production in breast cancer cells, leading to disruption of the Akt/HSP90 complex and subsequent Akt degradation, ultimately contributing to cell death. [] This ROS-dependent mechanism appears to be selective for cancer cells, sparing normal breast cells. []
Q7: What is the molecular formula and weight of this compound?
A7: this compound mesylate, the salt form commonly used in formulations, has the molecular formula C32H45N3O4S • CH4O3S and a molecular weight of 663.87 g/mol.
Q8: Have any computational studies been performed on this compound?
A8: Yes, computational methods have been used to develop a pharmacophore model for this compound interaction with the multidrug resistance protein 4 (MRP4/ABCC4). [] This model revealed that this compound shares a binding site with chemotherapeutic agents like adefovir and methotrexate, highlighting its potential as both a substrate and inhibitor of this transporter. []
Q9: What is the typical route of administration and absorption profile of this compound?
A10: this compound is administered orally. [, , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , ] Food significantly affects its absorption, with a high-calorie meal required to achieve optimal plasma concentrations. []
Q10: How is this compound metabolized in the body?
A11: this compound undergoes extensive metabolism in the liver, primarily by cytochrome P450 (CYP) enzymes, mainly CYP3A4 and CYP2C19. [, ] The major metabolite, this compound hydroxy-t-butylamide (M8), exhibits potent antiviral activity. [, , ]
Q11: What factors can influence the pharmacokinetics of this compound?
A11: Various factors can influence this compound pharmacokinetics, including age, co-administered medications, and liver function. For instance:
- Age: Children, especially infants, exhibit different pharmacokinetic profiles compared to adults, generally requiring higher doses to achieve similar drug exposure. [, , ]
- Co-administered drugs: Co-administration with other protease inhibitors can lead to significant drug interactions, altering the plasma concentrations of both drugs. [, , ] Similarly, drugs that induce or inhibit CYP3A4 can impact this compound metabolism. []
- Liver disease: Liver disease, especially moderate to severe, can impair this compound metabolism, leading to lower M8 formation and requiring dose adjustments. []
Q12: How do this compound plasma concentrations relate to its efficacy in treating HIV?
A13: Studies suggest a correlation between this compound plasma concentrations and virological treatment success in HIV-infected individuals. Patients with low this compound levels are at an increased risk of virologic failure. [] This finding highlights the importance of therapeutic drug monitoring to optimize treatment outcomes.
Q13: What types of in vitro models have been used to study this compound?
A14: Various in vitro models, including cell lines derived from different cancer types (e.g., breast, lung, ovarian, multiple myeloma) have been used to investigate this compound's anti-cancer effects. [, , , , , , ] These studies have provided insights into its mechanisms of action, such as induction of apoptosis, ER stress, and inhibition of specific signaling pathways. [, , , , , ]
Q14: What in vivo models have been employed to evaluate this compound's anti-cancer potential?
A15: this compound's in vivo efficacy has been assessed in various animal models, including mouse xenograft models of different cancer types. [, , , ] These studies have demonstrated that this compound can inhibit tumor growth in vivo, often in conjunction with markers of ER stress, autophagy, and apoptosis. [, , , ]
Q15: Have any clinical trials been conducted with this compound in cancer patients?
A15: Yes, several clinical trials have explored this compound's potential as an anti-cancer agent in humans. For instance:
- Phase I trials: These trials have primarily focused on establishing the maximum tolerated dose (MTD) and safety profile of this compound in patients with advanced solid tumors. [] Results indicate that this compound is generally well-tolerated at doses higher than those used for HIV treatment. []
- Phase I/II trials: A trial combining this compound with bortezomib in patients with advanced hematologic malignancies demonstrated promising activity, particularly in bortezomib-refractory multiple myeloma. []
Q16: What are the primary HIV protease mutations associated with this compound resistance?
A17: The D30N mutation is the most common primary resistance mutation selected for by this compound. [, ] While it confers minimal cross-resistance to other protease inhibitors, the emergence of L90M, often accompanied by secondary mutations, can lead to broad cross-resistance within the protease inhibitor class. []
Q17: How does this compound resistance impact subsequent treatment options for HIV?
A18: Interestingly, despite the emergence of D30N, many patients experiencing virologic failure on a this compound-containing regimen can successfully switch to another protease inhibitor and achieve viral suppression. [, ] This observation suggests that the development of cross-resistance, while possible, is not inevitable.
Q18: Is there any evidence of cross-resistance between this compound and other anti-cancer agents?
A19: While this compound demonstrates synergism with some chemotherapeutic agents, like bortezomib, [, ] specific information regarding cross-resistance patterns with other anti-cancer drugs is limited within the provided research papers.
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.


![[4-chloro-3-[5-methyl-3-[4-(2-pyrrolidin-1-ylethoxy)anilino]-1,2,4-benzotriazin-7-yl]phenyl] benzoate;hydrochloride](/img/structure/B1663545.png)
![2-[5-[3-[4-(2-bromo-5-fluorophenoxy)piperidin-1-yl]-1,2-oxazol-5-yl]tetrazol-2-yl]acetic acid](/img/structure/B1663549.png)
![3-[4-[[4-(2-chlorophenyl)-1,3-thiazol-2-yl]methoxy]-2-methylphenyl]propanoic acid](/img/structure/B1663568.png)
