Fingolimod
Descripción general
Descripción
Fingolimod is a sphingosine-1-phosphate receptor modulator used primarily in the treatment of relapsing-remitting multiple sclerosis. It was approved by the Food and Drug Administration in 2010 and is known for its ability to reduce the frequency of relapses and delay the progression of physical disability in patients with multiple sclerosis .
Aplicaciones Científicas De Investigación
Fingolimod has a wide range of scientific research applications:
Chemistry: Used in the synthesis of various derivatives for antibacterial and antifungal studies.
Biology: Studied for its effects on sphingosine-1-phosphate receptors and its role in immune modulation.
Industry: Utilized in the development of new therapeutic agents and in the study of sphingolipid metabolism.
Mecanismo De Acción
Target of Action
Fingolimod primarily targets the sphingosine 1-phosphate receptors (S1PRs) . These receptors play a crucial role in the immune system, particularly in the regulation of lymphocyte circulation .
Mode of Action
The active form of this compound, known as This compound phosphate , is a modulator of S1PRs . It binds to various S1PRs (1, 3, 4, and 5), exerting its mechanism of action in multiple sclerosis (MS) by preventing the egress of lymphocytes from lymphoid tissues . This results in a reduction of autoaggressive lymphocyte infiltration into the central nervous system (CNS) .
Biochemical Pathways
This compound affects several biochemical pathways. It exerts inhibitory effects on sphingolipid pathway enzymes, inhibits histone deacetylases, transient receptor potential cation channel subfamily M member 7 (TRMP7), cytosolic phospholipase A2α (cPLA2α), and reduces lysophosphatidic acid (LPA) plasma levels . It also activates protein phosphatase 2A (PP2A) and induces apoptosis, autophagy, cell cycle arrest, epigenetic regulations, macrophages M1/M2 shift, and enhances BDNF expression .
Pharmacokinetics
This compound is efficiently absorbed, with an oral bioavailability of >90%, and its absorption is unaffected by dietary intake . It has a half-life of 6-9 days, and steady-state pharmacokinetics are reached after 1-2 months of daily dosing . The long half-life of this compound, together with its slow absorption, means that this compound has a flat concentration profile over time with once-daily dosing . This compound is extensively metabolized, with biotransformation occurring via three main pathways .
Result of Action
The action of this compound results in a significant reduction in disease activity in patients with relapsing-remitting multiple sclerosis (RRMS) . By reducing the number of lymphocytes in circulation and the CNS, it suppresses inflammation and MS .
Action Environment
The action of this compound can be influenced by environmental factors. For instance, a study found an association between EBNA-1 IgG titers and multiple sclerosis progression in patients treated with this compound . This suggests that certain environmental factors, such as exposure to the Epstein-Barr virus, may influence the efficacy of this compound .
Análisis Bioquímico
Biochemical Properties
Fingolimod plays a significant role in biochemical reactions by interacting with sphingosine 1-phosphate receptors. It is phosphorylated to form this compound-phosphate, which binds to sphingosine 1-phosphate receptors 1, 3, 4, and 5. This binding leads to the internalization and degradation of these receptors, thereby modulating immune cell trafficking. This compound also interacts with other biomolecules, such as histone deacetylases, transient receptor potential cation channel subfamily M member 7, and cytosolic phospholipase A2α, influencing various biochemical pathways .
Cellular Effects
This compound exerts multiple effects on various cell types and cellular processes. It influences cell signaling pathways by modulating sphingosine 1-phosphate receptor activity, leading to altered lymphocyte trafficking and reduced inflammation. This compound also affects gene expression by inhibiting histone deacetylases, resulting in changes in the expression of genes involved in immune responses and cell cycle regulation. Additionally, this compound impacts cellular metabolism by modulating oxidative phosphorylation, glycolysis, and the pentose phosphate pathway .
Molecular Mechanism
The molecular mechanism of action of this compound involves its phosphorylation to this compound-phosphate, which binds to sphingosine 1-phosphate receptors. This binding leads to receptor internalization and degradation, reducing the availability of these receptors on the cell surface. This compound also inhibits histone deacetylases, leading to changes in gene expression. Furthermore, this compound modulates various signaling pathways, including the retrograde endocannabinoid signaling and autophagy pathways, contributing to its therapeutic effects .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of this compound change over time. This compound is stable under physiological conditions, but its effects on cellular function can vary depending on the duration of exposure. Long-term studies have shown that this compound can induce metabolic reprogramming and modulate neuroinflammation pathways over extended periods. These temporal effects are crucial for understanding the long-term therapeutic potential of this compound .
Dosage Effects in Animal Models
The effects of this compound vary with different dosages in animal models. At low doses, this compound effectively modulates immune cell trafficking and reduces inflammation. At higher doses, this compound can induce toxic effects, including bradycardia and macular edema. These dosage-dependent effects highlight the importance of optimizing the dosage of this compound for therapeutic use .
Metabolic Pathways
This compound is involved in several metabolic pathways, including the sphingolipid metabolism pathway. It is phosphorylated by sphingosine kinase to form this compound-phosphate, which then interacts with sphingosine 1-phosphate receptors. This compound also affects other metabolic pathways, such as oxidative phosphorylation, glycolysis, and the pentose phosphate pathway, leading to changes in metabolic flux and metabolite levels .
Transport and Distribution
This compound is transported and distributed within cells and tissues through various mechanisms. It is taken up by cells via passive diffusion and is phosphorylated to form this compound-phosphate. This compound-phosphate is then transported to different cellular compartments, where it interacts with sphingosine 1-phosphate receptors. This compound also binds to plasma proteins, influencing its distribution and accumulation in tissues .
Subcellular Localization
This compound is localized in various subcellular compartments, including the cytoplasm, nucleus, and cell membrane. Its subcellular localization is influenced by post-translational modifications, such as phosphorylation, which direct it to specific compartments. This compound’s activity and function are affected by its localization, as it interacts with different biomolecules in various cellular compartments .
Métodos De Preparación
Synthetic Routes and Reaction Conditions: Fingolimod can be synthesized starting from n-octylbenzene and 3-nitropropionic acid. The synthetic route involves a sequence of reactions including Friedel-Crafts acylation, reduction, and double Henry reaction, followed by hydrogenation. This method yields this compound with a yield of 31% and an overall atom economy of 82.7% .
Industrial Production Methods: In industrial settings, this compound is often synthesized using a reverse phase liquid chromatography method. This involves the use of a phosphate buffer, methanol, and acetonitrile as eluents, with separation achieved using a specific column and temperature conditions .
Análisis De Reacciones Químicas
Types of Reactions: Fingolimod undergoes various chemical reactions, including oxidation, reduction, and substitution. It forms charge transfer complexes with different electron acceptor reagents, such as 7,7,8,8-tetracyanoquinodimethane, tetrachloro 1,4-benzoquinone, and tetracyanoethylene .
Common Reagents and Conditions: The reactions typically involve reagents like orthophosphoric acid, methanol, and acetonitrile under controlled temperature and pH conditions .
Major Products: The major products formed from these reactions include stable anions that can be measured spectrophotometrically .
Comparación Con Compuestos Similares
Dimethyl fumarate: Another oral disease-modifying treatment for multiple sclerosis, comparable in effectiveness to Fingolimod.
Sphingosine: A naturally occurring sphingoid base with antimicrobial properties.
Uniqueness: this compound is unique due to its specific mechanism of action involving sphingosine-1-phosphate receptor modulation, which is distinct from other immunosuppressants. Its ability to sequester lymphocytes and prevent their egress from lymphoid tissues sets it apart from other treatments .
Propiedades
IUPAC Name |
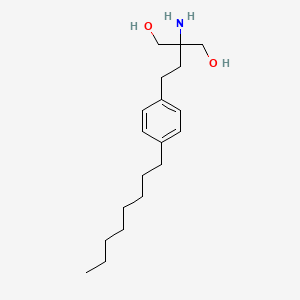
2-amino-2-[2-(4-octylphenyl)ethyl]propane-1,3-diol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C19H33NO2/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-22/h9-12,21-22H,2-8,13-16,20H2,1H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
KKGQTZUTZRNORY-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCCCCCCCC1=CC=C(C=C1)CCC(CO)(CO)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C19H33NO2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID40167363 | |
| Record name | Fingolimod | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID40167363 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
307.5 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
Soluble | |
| Record name | Fingolimod | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08868 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Mechanism of Action |
Sphingosine‐1‐phosphate (S1P) is an important phospholipid that binds to various G‐protein‐coupled receptor subtypes, which can be identified as S1P1–5R. S1P and the receptors it binds to perform regular functions in the immune, cardiovascular, pulmonary, and nervous systems. S1P can be expressed ubiquitously, playing an important role in regulating inflammation. S1P1R, S1P2R, and S1P3R receptors can be found in the cardiovascular, immune, and central nervous systems. S1P4R is found on lymphocytic and hematopoietic cells, while S1P5R expression is found only on the spleen (on natural killer cells) or in the central nervous system. The active form of the drug, fingolimod phosphate, is a sphingosine 1-phosphate receptor modulator that exerts its mechanism of action in MS by binding to various sphingosine 1-phosphate receptors (1, 3, 4, and 5). It suppresses the exit of lymphocytes from lymph nodes, leading to a lower level of lymphocytes circulating in the peripheral circulation. This reduces the inflammation that is associated with MS. The mechanism of action of fingolimod is not fully understood but may be related to reduced lymphocyte circulation into the central nervous system. Immune modulating treatment such as fingolimod is not typically employed for SARS-CoV-2 pneumonia. Despite this, with the tissue findings of pulmonary edema and hyaline membrane formation, the timely use of immune modulators such as fingolimod can be considered to prevent acute respiratory distress syndrome (ARDS) associated with COVID-19. | |
| Record name | Fingolimod | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08868 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
162359-55-9 | |
| Record name | Fingolimod | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=162359-55-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Fingolimod [INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0162359559 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Fingolimod | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08868 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Fingolimod | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID40167363 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | FINGOLIMOD | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/3QN8BYN5QF | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Melting Point |
102-107 | |
| Record name | Fingolimod | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08868 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Synthesis routes and methods I
Procedure details







Synthesis routes and methods II
Procedure details





Synthesis routes and methods III
Procedure details





Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary mechanism of action of Fingolimod in the context of multiple sclerosis?
A: this compound, after undergoing phosphorylation in vivo to its active metabolite, this compound-phosphate, functions as a sphingosine 1-phosphate receptor (S1PR) modulator. This modulation specifically targets the S1P1 subtype of these receptors, which are predominantly found on lymphocytes. By binding to S1P1, this compound-phosphate effectively prevents the egress of these lymphocytes from lymph nodes, reducing their circulation and, consequently, their trafficking into the central nervous system (CNS). This modulation of lymphocyte migration is believed to be a key mechanism by which this compound exerts its therapeutic effects in relapsing multiple sclerosis (MS). []
Q2: How does this compound's activity differ from other disease-modifying therapies for multiple sclerosis?
A: Unlike many other disease-modifying therapies for MS, which are administered parenterally, this compound is orally active. Additionally, its unique mechanism of action involves modulating S1PRs, which are found not only on lymphocytes but also within the CNS, specifically on glial cells such as astrocytes and oligodendrocytes. [] This suggests that this compound's efficacy might stem from a combination of immunomodulatory effects on lymphocyte trafficking and potential direct effects on neural cells, distinguishing it from other MS treatments. []
Q3: Does this compound directly protect myelin from damage in MS models?
A: Studies utilizing a cuprizone-induced demyelination model in mice suggest that while this compound does not directly prevent demyelination, it demonstrates a neuroprotective effect. [] Despite extensive demyelination observed in both this compound-treated and control groups, a significant reduction in axonal transection markers was observed in this compound-treated mice. [] This indicates that this compound may not halt the initial myelin loss, but it could play a role in protecting the underlying axons from damage during active demyelination. []
Q4: Beyond its impact on lymphocyte trafficking, what other potential mechanisms of action are being investigated for this compound in MS?
A: Research suggests that this compound might exert neuroprotective and reparative effects within the CNS. [] Preclinical studies using a mouse model of Krabbe's disease, a neurodegenerative disorder characterized by demyelination, have shown that this compound can rescue myelin levels, regulate glial cell reactivity, and increase lifespan. [] These findings, along with observations in MS models, point towards additional mechanisms involving S1PR modulation in the CNS that could contribute to this compound's efficacy. [, ]
Q5: How does this compound's impact on cytokine production in immune cells contribute to its potential neuroprotective effects in MS?
A: In vitro studies using peripheral blood mononuclear cells (PBMCs) from relapsing-remitting MS patients demonstrated that this compound significantly increases the expression of specific cytokines, chemokines, and growth factors. [] These include IL-6, IL-10, IL-17A, CXCL1, and FGFb, all of which have been implicated in neuroprotective and regenerative processes within the CNS. [] For instance, these molecules are involved in promoting neuronal regeneration, enhancing neuronal survival, and activating neuronal progenitor cells. []
Q6: What is the significance of this compound's ability to cross the blood-brain barrier in the context of MS treatment?
A: this compound readily crosses the blood-brain barrier, and its active metabolite, this compound-phosphate, can be formed within the CNS. [] This characteristic is particularly significant because it allows this compound to potentially exert direct effects on neural cells, in addition to its peripheral effects on lymphocyte trafficking. [] This dual action distinguishes it from other MS therapies that primarily target peripheral immune responses and highlights its potential for broader therapeutic benefits. []
Q7: How is the pharmacokinetic profile of this compound-phosphate characterized?
A: Analysis of data from Phase 1 studies, utilizing a population model, demonstrated that the concentration-time course of this compound-phosphate, the active metabolite of this compound, following oral administration can be described by a two-compartment model. [] This model incorporates first-order apparent formation and elimination, a lag time in the apparent formation, and dose-dependent relative bioavailability and apparent central volume of distribution. []
Q8: Have there been any observed relationships between demographic factors and this compound pharmacokinetics?
A: Population pharmacokinetic modeling has indicated that both body weight and ethnicity may influence the disposition of this compound-phosphate. [] While the model suggests that dose adjustments based on body weight may not be necessary, further investigations are needed to clarify the influence of ethnicity on this compound pharmacokinetics. []
Q9: What is the impact of this compound on vaccination responses in patients with MS?
A: Research indicates that this compound treatment can lead to a reduced immune response to both novel and recall antigens in vaccines. [] A study investigating responses to influenza and tetanus toxoid vaccines in MS patients found that while most this compound-treated patients could mount immune responses and achieve seroprotection, the response rates were significantly lower compared to placebo-treated patients. [] This highlights the need for careful consideration and monitoring of vaccination strategies in patients receiving this compound therapy. []
Q10: What are the implications of this compound's impact on T-cell populations and activation for antiviral immunity in MS patients?
A: this compound therapy leads to a substantial reduction in CD3+ T cells, particularly affecting naive and central memory T cells, while increasing effector memory T cell populations. [] Although the expression of T-cell activation markers upon viral stimulation seems unaffected by this compound, the absolute number of proliferating cells and those producing antiviral cytokines, such as IFN-γ, is reduced. [] This suggests a potential for impaired antiviral immunity, although further research is needed to fully elucidate the clinical implications of these findings. []
Q11: Are there any specific safety concerns associated with the discontinuation of this compound in MS patients?
A: Discontinuation of this compound can potentially lead to a rebound of disease activity in some patients with MS. [, ] Case reports and observational studies have highlighted instances of severe relapses and increased inflammatory activity shortly after this compound cessation. [, ] This rebound effect underscores the importance of careful monitoring and potentially implementing strategies to mitigate disease reactivation when transitioning patients off this compound therapy. [, ]
Q12: What are the potential implications of combining this compound with radiation therapy in the context of glioblastoma treatment?
A: Preclinical studies in mice and a pilot study in humans suggest that combining this compound with radiation therapy is feasible and safe. [] The rationale behind this approach lies in this compound's ability to induce transient lymphopenia by sequestering lymphocytes in lymphoid tissues, potentially protecting them from the damaging effects of radiation. [] While this combination therapy led to more pronounced lymphopenia, it was well-tolerated, and lymphocyte counts recovered in both mice and human subjects. [] Further research is warranted to determine the clinical efficacy of this approach. []
Q13: Can you provide an overview of this compound’s development history and key milestones?
A: this compound (FTY720), a sphingosine-1-phosphate receptor modulator, marked a significant advancement as the first oral disease-modifying therapy approved for relapsing multiple sclerosis (MS). [] Its journey began with preclinical studies demonstrating its efficacy in the experimental autoimmune encephalomyelitis (EAE) model of MS. [] Following promising results in Phase 2 and 3 clinical trials, this compound received approval from the US Food and Drug Administration in 2010 for reducing relapses and delaying disability progression in relapsing forms of MS. [, , ] This approval marked a turning point in MS treatment, offering patients a more convenient oral alternative to injectable therapies and expanding treatment options. [, ]
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.