Metildopa
Descripción general
Descripción
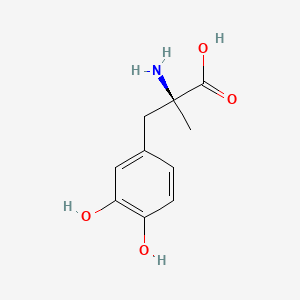
Metildopa, químicamente conocido como L-α-Metil-3,4-dihidroxi-fenilalanina, es un medicamento utilizado principalmente para controlar la presión arterial alta. Se introdujo por primera vez en 1960 y es reconocido por su efectividad en el tratamiento de la hipertensión, particularmente en mujeres embarazadas. This compound está incluida en la Lista de Medicamentos Esenciales de la Organización Mundial de la Salud debido a su importancia en la atención médica .
Mecanismo De Acción
Metildopa es un agonista alfa-2 adrenérgico de acción central. Se metaboliza en el cuerpo a α-metil norepinefrina, que estimula los receptores alfa-2 adrenérgicos en el sistema nervioso central. Esto lleva a una reducción de la actividad del sistema nervioso simpático, lo que resulta en una disminución de la resistencia vascular y una menor presión arterial . This compound también inhibe la descarboxilación de la dopa a dopamina, contribuyendo a sus efectos antihipertensivos .
Aplicaciones Científicas De Investigación
Metildopa tiene una amplia gama de aplicaciones en investigación científica:
Medicina: this compound se utiliza ampliamente en el tratamiento de la hipertensión, particularmente en mujeres embarazadas.
Industria: this compound se utiliza en la industria farmacéutica para la producción de medicamentos antihipertensivos.
Análisis Bioquímico
Biochemical Properties
Methyldopa works by binding to alpha (α)-2 adrenergic receptors as an agonist, leading to the inhibition of adrenergic neuronal outflow and reduction of vasoconstrictor adrenergic signals . Methyldopa exists in two isomers D-α-methyldopa and L-α-methyldopa, which is the active form .
Cellular Effects
Methyldopa has a significant impact on various types of cells and cellular processes. It influences cell function by reducing the activity of the sympathetic nervous system . This reduction in activity leads to a decrease in blood pressure, making methyldopa an effective treatment for hypertension .
Molecular Mechanism
The molecular mechanism of methyldopa involves its binding to alpha (α)-2 adrenergic receptors as an agonist . This binding leads to the inhibition of adrenergic neuronal outflow and a reduction in vasoconstrictor adrenergic signals . This mechanism is how methyldopa exerts its effects at the molecular level.
Temporal Effects in Laboratory Settings
The plasma half-life of methyldopa is approximately 105 minutes . Following intravenous injection, the plasma half-life of methyldopa ranges from 90 to 127 minutes . This indicates that the effects of methyldopa can change over time in laboratory settings.
Dosage Effects in Animal Models
While specific studies on the dosage effects of methyldopa in animal models are limited, it is known that methyldopa is used to manage hypertension in clinical settings
Metabolic Pathways
Methyldopa is metabolised by dopamine hydroxylase (DBH) to its active metabolite β-methylnorepinephrine . This metabolite acts as an agonist at the pre-synaptic 2 adrenergic α α receptors in the brainstem, resulting in a reduced adrenergic neuronal outflow through the peripheral nervous system causing vasodilation and reduced blood pressure .
Transport and Distribution
While specific information on the transport and distribution of methyldopa within cells and tissues is limited, it is known that methyldopa works by binding to alpha (α)-2 adrenergic receptors as an agonist . This binding leads to the inhibition of adrenergic neuronal outflow and reduction of vasoconstrictor adrenergic signals .
Subcellular Localization
Given that methyldopa is a centrally acting sympatholytic agent and an antihypertensive agent , it is likely that it interacts with receptors and other structures within cells to exert its effects
Métodos De Preparación
Rutas sintéticas y condiciones de reacción: Metildopa se puede sintetizar a través de varios métodos. Una ruta sintética común implica la reacción de 3,4-dihidroxibenzaldehído con nitrometahano para formar 3,4-dihidroxi-β-nitroestireno. Este intermedio se reduce luego a 3,4-dihidroxi-fenilacetaldehído, que se somete a aminación reductora con metilamina para producir this compound .
Métodos de producción industrial: En entornos industriales, la this compound a menudo se produce mediante un proceso de secado fluidizado. Esto implica polimerizar la this compound con alcohol esteárico y dióxido de silicio, lo que da como resultado una composición con mejor fluidez y compresibilidad. Este método mejora la estabilidad y la vida útil del producto final .
Análisis De Reacciones Químicas
Tipos de reacciones: Metildopa se somete a varias reacciones químicas, incluida la oxidación, la reducción y la sustitución.
Reactivos y condiciones comunes:
Reducción: La reducción de this compound se puede lograr utilizando gas hidrógeno en presencia de un catalizador de paladio.
Principales productos formados: Los principales productos formados a partir de estas reacciones incluyen varios derivados de this compound, como la α-metil norepinefrina, que es un metabolito activo de la this compound .
Comparación Con Compuestos Similares
Metildopa a menudo se compara con otros agentes antihipertensivos como lisinopril, amlodipina, labetalol, nifedipina, hidralazina, clonidina y furosemida .
Singularidad:
This compound vs. Lisinopril: Si bien ambos se utilizan para controlar la hipertensión, la this compound es preferible en mujeres embarazadas debido a su perfil de seguridad, mientras que el lisinopril es un inhibidor de la enzima convertidora de angiotensina con un mecanismo de acción diferente.
This compound vs. Amlodipina: Amlodipina es un bloqueador de los canales de calcio, mientras que la this compound actúa centralmente sobre los receptores alfa-2 adrenérgicos.
This compound vs. Labetalol: Labetalol es un bloqueador beta con actividad de bloqueo alfa, lo que lo hace adecuado para emergencias hipertensivas, mientras que la this compound se utiliza principalmente para el control de la hipertensión crónica.
Compuestos similares:
- Lisinopril
- Amlodipina
- Labetalol
- Nifedipina
- Hidralazina
- Clonidina
- Furosemida
El mecanismo de acción único de la this compound y su perfil de seguridad, especialmente en el embarazo, la distinguen de otros agentes antihipertensivos.
Propiedades
IUPAC Name |
(2S)-2-amino-3-(3,4-dihydroxyphenyl)-2-methylpropanoic acid | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C10H13NO4/c1-10(11,9(14)15)5-6-2-3-7(12)8(13)4-6/h2-4,12-13H,5,11H2,1H3,(H,14,15)/t10-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
CJCSPKMFHVPWAR-JTQLQIEISA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(CC1=CC(=C(C=C1)O)O)(C(=O)O)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C[C@](CC1=CC(=C(C=C1)O)O)(C(=O)O)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C10H13NO4 | |
| Record name | METHYL DOPA | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20642 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
27289-76-5 | |
| Record name | L-Tyrosine, 3-hydroxy-α-methyl-, homopolymer | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=27289-76-5 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
DSSTOX Substance ID |
DTXSID5023295 | |
| Record name | (-)-Methyldopa | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5023295 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
211.21 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Methyl dopa appears as colorless or almost colorless crystals or white to yellowish-white fine powder. Almost tasteless. In the sesquihydrate form. pH (saturated aqueous solution) about 5.0. (NTP, 1992), Solid | |
| Record name | METHYL DOPA | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20642 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Methyldopa | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0011754 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
>31.7 [ug/mL] (The mean of the results at pH 7.4), 1 to 10 mg/mL at 73 °F (NTP, 1992), Sol in water @ 25 °C: approx 10 mg/ml /D-form/, Sol in water @ 25 °C: approx 18 mg/ml /DL-form/, In water @ 25 °C: about 10 mg/ml; practically insol in common org solvents; sol in dil mineral acids, Soluble in isopropanol, ethanol, and water., 10mg/mL at 25 °C | |
| Record name | SID8139902 | |
| Source | Burnham Center for Chemical Genomics | |
| URL | https://pubchem.ncbi.nlm.nih.gov/bioassay/1996#section=Data-Table | |
| Description | Aqueous solubility in buffer at pH 7.4 | |
| Record name | METHYL DOPA | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20642 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Methyldopa | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00968 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | METHYLDOPA | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/218 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Methyldopa | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0011754 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
The exact mechanism of methyldopa is not fully elucidated; however, the main mechanisms of methyldopa involve its actions on alpha-adrenergic receptor and the aromatic L-amino acid decarboxylase enzyme, to a lesser extent. The sympathetic outflow is regulated by alpha (α)-2 adrenergic receptors and imidazoline receptors expressed on adrenergic neurons within the rostral ventrolateral medulla. Methyldopa is metabolized to α‐methylnorepinephrine via dopamine beta-hydroxylase activity and, consequently, alpha-methylepinephrine via phenylethanolamine-N-methyltransferase activity. Mediating the therapeutic effects of methyldopa, α‐methylnorepinephrine and α-methylepinephrine active metabolites are agonists at presynaptic alpha-2 adrenergic receptors in the brainstem. Stimulating alpha-2 adrenergic receptors results in the inhibition of adrenergic neuronal outflow and attenuation of norepinephrine release in the brainstem. Consequently, the output of vasoconstrictor adrenergic signals to the peripheral sympathetic nervous system is reduced, leading to a reduction in blood pressure. The L-isomer of alpha-methyldopa also reduces blood pressure by inhibiting aromatic L-amino acid decarboxylase, also known as DOPA decarboxylase, which is an enzyme responsible for the syntheses of dopamine and serotonin. Inhibiting this enzyme leads to depletion of biogenic amines such as norepinephrine. However, inhibition of aromatic L-amino acid decarboxylase plays a minimal role in the blood-pressure‐lowering effect of methyldopa., METHYLDOPA...HAS HYPOTENSIVE ACTION INDEPENDENT OF ITS ANTIADRENERGIC ACTIONS; THIS IS PROBABLY PARTLY CENTRAL DEPRESSANT ACTION @ VASOMOTOR CENTER & PARTLY PERIPHERAL ACTION OF UNKNOWN MECHANISM., ... Alpha-methylnorepinephrine acts in the brain to inhibit adrenergic neuronal outflow from the brainstem, and this central effect is principally responsible for its antihypertensive action., IN CONSCIOUS RENAL HYPERTENSIVE RATS ALPHA-METHYLDOPA PRODUCED A LONG-LASTING FALL IN BLOOD PRESSURE WHICH WAS PARTIALLY ATTENUATED BY PRETREATMENT WITH NALTREXONE (5 MG/KG SC). PRETREATMENT WITH ANTISERUM TO BETA-ENDORPHIN APPLIED LOCALLY, ALSO BLOCKED THE DEPRESSOR RESPONSE. THESE RESULTS SUGGEST THAT THE FALL IN BLOOD PRESSURE OBSERVED AFTER ALPHA-METHYLDOPA AND ITS ACTIVE METABOLITE ALPHA-METHYLNORADRENALINE INVOLVES A BETA-ENDORPHIN LIKE PEPTIDE; A POSSIBLE SITE OF ACTION IS THE NUCLEUS TRACTUS SOLITARII., A REVIEW ON THE MECHANISM OF ACTION. | |
| Record name | Methyldopa | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00968 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | METHYLDOPA | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/218 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Minute, anhyd crystals from methanol, WHITE TO YELLOWISH WHITE, FINE POWDER, WHICH MAY CONTAIN FRIABLE LUMPS | |
CAS No. |
555-30-6, 41372-08-1 | |
| Record name | METHYL DOPA | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20642 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Methyldopa | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=555-30-6 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Methyldopa [INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000555306 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Methyldopa | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00968 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | methyldopa | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=760080 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | L-Tyrosine, 3-hydroxy-.alpha.-methyl- | |
| Source | EPA Chemicals under the TSCA | |
| URL | https://www.epa.gov/chemicals-under-tsca | |
| Description | EPA Chemicals under the Toxic Substances Control Act (TSCA) collection contains information on chemicals and their regulations under TSCA, including non-confidential content from the TSCA Chemical Substance Inventory and Chemical Data Reporting. | |
| Record name | (-)-Methyldopa | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5023295 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Methyldopa | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.008.264 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | METHYLDOPA ANHYDROUS | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/M4R0H12F6M | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | METHYLDOPA | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/218 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Methyldopa | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0011754 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
572 °F approximately (decomposes) (NTP, 1992), >300, 300 °C | |
| Record name | METHYL DOPA | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20642 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Methyldopa | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00968 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Methyldopa | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0011754 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
A: α-Methyldopa is a centrally acting antihypertensive agent. It is converted to α-methylnorepinephrine in the brain, which then acts as an agonist at central α2-adrenergic receptors. [] This activation reduces sympathetic outflow from the brain, leading to a decrease in peripheral vascular resistance and blood pressure. [, , ]
A: While its primary action is central, α-Methyldopa does have some peripheral effects. Studies in rabbits suggest that about 70% of its action is central, while 30% is attributed to peripheral mechanisms. [] Peripherally, α-methylnorepinephrine can act as a false neurotransmitter, competing with norepinephrine at sympathetic nerve endings. This can contribute to its antihypertensive effect, particularly at lower frequencies of sympathetic nerve stimulation. [, ]
A: Research indicates that some of α-Methyldopa's central effects might be mediated through serotonin pathways. Experiments in rabbits showed that destroying serotonergic neurons with 5,6-dihydroxytryptamine attenuated the effects of centrally administered α-Methyldopa on mean arterial pressure and heart rate by approximately 50%. []
A: Studies suggest that β-adrenergic receptor stimulation might play a role in the overall effects of α-Methyldopa. Metabolites of α-Methyldopa have shown high potency in competing for β1 receptors in the forebrain. Additionally, (-)-erythro-α-methylepinephrine, a α-Methyldopa metabolite, displayed a higher potency than (-)-epinephrine, (-)-norepinephrine, and (-)-erythro-α-methylnorepinephrine in competing for β2 receptors on human lymphocytes. []
A: The molecular formula of α-Methyldopa sesquihydrate is C10H13NO4 · 1½H2O. Its molecular weight is 238.24 g/mol. []
A: α-Methyldopa itself is a prodrug, meaning it is metabolized in the body to its active form, α-methylnorepinephrine. This metabolite is structurally similar to norepinephrine, allowing it to interact with adrenergic receptors. The α-methyl group is crucial for its central activity and reduces its potency at peripheral adrenergic receptors compared to norepinephrine. [, ]
A: One challenge in formulating α-Methyldopa is its susceptibility to oxidation. [] Exposure to air, light, and high pH can degrade the drug, leading to changes in color and efficacy.
A: α-Methyldopa is well absorbed after oral administration. It is primarily metabolized in the liver and central nervous system to α-methyldopamine and α-methylnorepinephrine. [, , ]
A: α-Methyldopa and its metabolites are mainly excreted in the urine. [, , ] Studies in neonates showed that α-Methyldopa is readily metabolized to α-methyldopa sulfate, and both are excreted slowly compared to adults, resulting in a longer half-life. []
A: Studies indicate a potential for dose-dependent metabolism of α-Methyldopa. Research in Parkinson's disease patients revealed that the proportion of the dose excreted as vanillacetic acid (VLA) increased linearly with increasing α-Methyldopa dosage. This suggests a potential induction of the transaminase enzyme involved in its metabolism. []
A: Yes, α-Methyldopa has been shown to interact with iron supplementation. α-Methyldopa can form complexes with both ferrous and ferric iron. This interaction is pH-dependent, with complex formation being faster at higher pH levels. The tight binding of ferric iron to α-Methyldopa can potentially alter the drug's biodistribution. Furthermore, under the acidic conditions of the stomach, redox cycling can occur, leading to both catechol oxidation and the production of potentially harmful hydroxyl radicals. Therefore, caution is advised when administering α-Methyldopa concurrently with ferrous sulfate. []
A: α-Methyldopa consistently demonstrated antihypertensive effects in various animal models. In spontaneously hypertensive rats (SHR), both long-term intravenous infusions and prolonged oral administration of α-Methyldopa significantly reduced mean arterial pressure. [, ] These effects are consistent with its mechanism of action, primarily involving the central nervous system.
A: Research suggests that α-Methyldopa might have a beneficial effect on cardiac hypertrophy associated with hypertension. In SHR, prolonged treatment with α-Methyldopa resulted in significantly reduced cardiac masses and heart weight-to-body weight ratios compared to untreated controls. Interestingly, this effect was not observed with clonidine, another centrally acting antihypertensive. [] Furthermore, α-Methyldopa also reduced cardiac mass in normotensive Wistar Kyoto rats without significantly affecting systemic hemodynamics, suggesting a potential direct effect on cardiac hypertrophy beyond blood pressure reduction. []
A: α-Methyldopa has been associated with several side effects, including drowsiness, dry mouth, headache, and dizziness. [, , ] In rare cases, it can also cause more serious adverse effects like liver damage and hemolytic anemia. [, , ]
A: The impact of α-Methyldopa on GH secretion appears to be more complex and potentially time-dependent. While short-term α-Methyldopa treatment in hypertensive patients did not significantly affect GH levels during an insulin stimulation test, there are suggestions that long-term treatment might have different effects. [] One hypothesis is that the time course of α-Methyldopa's effect on GH secretion might be related to the gradual substitution of endogenous catecholamines with α-Methyldopa metabolites within the brain. []
A: Liquid chromatography (LC) is a commonly used method for determining α-Methyldopa concentrations in both pharmaceutical formulations and biological samples. [] Reverse-phase C18 columns, coupled with UV detection, have been successfully employed for this purpose. This method offers high sensitivity and can also be used to quantify α-Methyldopa in combination with other drugs like hydrochlorothiazide and chlorothiazide. []
A: Yes, spectrophotometric methods based on charge-transfer complexation can be used for the determination of α-Methyldopa in pharmaceutical formulations. [] One such method utilizes bromanil as a complexing agent, resulting in a green-colored product with maximum absorbance at 738 nm. This method offers a simple, rapid, and cost-effective approach for α-Methyldopa quantification. []
A: α-Methyldopa has been associated with the development of autoimmune hemolytic anemia in some patients, although this is a rare side effect. [, ] The mechanism is not fully understood, but it is thought to involve the production of autoantibodies against red blood cells. []
A: Research suggests that α-Methyldopa metabolism might involve COMT. Experiments using dopacetamide, a COMT inhibitor, revealed alterations in the urinary excretion profile of α-Methyldopa metabolites in rats. Dopacetamide administration led to an increase in the urinary content of 14C-3,4-dihydroxyphenylacetic acid and 14C-dopamine, while decreasing 14C-3-methoxytyramine levels. [] This observation suggests that COMT might play a role in the O-methylation pathway of α-Methyldopa metabolism.
ANone: Several alternative antihypertensive medications are available, each with its own mechanism of action and side effect profile. Some commonly used alternatives include:
- Beta-blockers (e.g., metoprolol, propranolol, labetalol): These drugs block the effects of adrenaline on the heart and blood vessels, leading to a decrease in heart rate and blood pressure. [, , , ]
- Calcium channel blockers (e.g., nifedipine, diltiazem): These medications relax and widen blood vessels by blocking the entry of calcium into muscle cells lining the vessel walls. [, ]
- ACE inhibitors (e.g., captopril): These drugs block the action of angiotensin-converting enzyme (ACE), an enzyme that produces angiotensin II, a potent vasoconstrictor. []
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.