Vancomicina
Descripción general
Descripción
La vancomicina es un antibiótico glucopéptido utilizado principalmente para tratar infecciones bacterianas graves causadas por bacterias Gram-positivas, incluyendo Staphylococcus aureus resistente a la meticilina (SARM) y Clostridium difficile . Se aisló por primera vez de una muestra de suelo en Borneo en 1956 y desde entonces se ha convertido en una herramienta crucial en la lucha contra las infecciones resistentes a los antibióticos .
Mecanismo De Acción
La vancomicina actúa inhibiendo la síntesis de la pared celular en las bacterias. Se une al extremo D-alanil-D-alanina de las unidades precursoras de la pared celular, evitando su incorporación a la pared celular y debilitando así la pared celular bacteriana, lo que lleva a la lisis y la muerte celular . Este mecanismo lo hace altamente eficaz contra las bacterias Gram-positivas .
Aplicaciones Científicas De Investigación
La vancomicina tiene una amplia gama de aplicaciones en la investigación científica:
Análisis Bioquímico
Biochemical Properties
Vancomycin acts by binding to the D-alanyl-D-alanine moieties of the NAM/NAG-peptides, preventing the incorporation of these subunits into the peptidoglycan matrix, which forms the major structural component of Gram-positive bacterial cell walls . This interaction is non-covalent and results in the inhibition of cell wall synthesis . Vancomycin’s activity is concentration-independent, also referred to as “time-dependent,” and its clinical activity can be affected by factors such as variable tissue distribution, inoculum size, and emerging resistance .
Cellular Effects
Vancomycin primarily targets bacterial cells, where it disrupts cell wall synthesis, leading to cell death . It has been shown to have variable effects on different types of cells. For example, it has been found to reduce cell viability by 20% to 30% with increasing concentration up from 0.2 mg/mL . It also induces oxidative stress, as indicated by a reduction in the antioxidant activity of SOD2 and an increase in the MDA level .
Molecular Mechanism
Vancomycin exerts its effects at the molecular level by forming an intricate network of hydrogen bonds with the D-Ala-D-Ala region of Lipid II, interfering with the peptidoglycan layer maturation process . This binding prevents the incorporation of N-acetylmuramic acid (NAM) and N-acetylglucosamine (NAG)-peptide subunits into the peptidoglycan matrix .
Temporal Effects in Laboratory Settings
The pharmacokinetic profile of vancomycin is complex and can be characterized by either a 2- or 3-compartment pharmacokinetic profile . In patients with normal creatinine clearance, vancomycin has an α-distribution phase of approximately 30 minutes to 1 hour and a β-elimination half-life of 6–12 hours . Over time, the effects of vancomycin can change due to factors such as the development of resistance .
Dosage Effects in Animal Models
In animal models, the effects of vancomycin can vary with different dosages. For instance, in beagle dogs, vancomycin is administered intravenously at a dose rate of 20 mg/kg over a 1-hour period at 12-hour intervals . The effects of vancomycin in animal models can also be influenced by factors such as the animal’s health status and the presence of other medications.
Metabolic Pathways
Vancomycin is not appreciably metabolized and is eliminated primarily via the renal route, with more than 80% to 90% recovered unchanged in urine within 24 hours after administration of a single dose . It has been suggested that vancomycin disrupts amino acids metabolism, fatty acids biosynthesis, energy metabolism, and lipid metabolism .
Transport and Distribution
Vancomycin is administered intravenously and has a volume of distribution of 0.4–1 L/kg . It penetrates into most body spaces, although the concentrations obtained are variable and somewhat dependent on the degree of inflammation present .
Subcellular Localization
Vancomycin accumulates in lysosomes of proximal tubular cells throughout 10 days of treatment . The subcellular localization of vancomycin in the renal cortices of rats was determined with ultrathin sections by immunogold labeling .
Métodos De Preparación
La vancomicina se produce mediante la fermentación de la bacteria Amycolatopsis orientalis . La producción industrial implica varios pasos:
Fermentación: Amycolatopsis orientalis se cultiva en un medio rico en nutrientes para producir this compound.
Extracción: El antibiótico se extrae del caldo de fermentación utilizando solventes orgánicos.
Purificación: La cromatografía líquida de alta resolución (HPLC) se utiliza comúnmente para purificar la this compound.
Análisis De Reacciones Químicas
La vancomicina experimenta varias reacciones químicas, incluyendo:
Oxidación y Reducción: Estas reacciones pueden modificar los grupos funcionales en la molécula de this compound, alterando potencialmente su actividad.
Reacciones de Sustitución: Los reactivos comunes incluyen acetonitrilo y metanol, utilizados en métodos cromatográficos.
Productos Principales: El producto principal es la this compound purificada, que conserva sus propiedades antibióticas.
Comparación Con Compuestos Similares
La vancomicina a menudo se compara con otros antibióticos, como:
Linezolid: Una oxazolidinona sintética que inhibe la síntesis de proteínas.
Daptomicina: Un lipopeptido que altera la función de la membrana celular.
Tigeciclina: Una glicilciclina que inhibe la síntesis de proteínas.
Teicoplanina: Otro glucopéptido con un mecanismo de acción similar.
La singularidad de la this compound radica en su capacidad para tratar infecciones graves causadas por bacterias resistentes a los antibióticos, lo que la convierte en una herramienta vital en la medicina moderna .
Propiedades
Número CAS |
1404-90-6 |
|---|---|
Fórmula molecular |
C66H75Cl2N9O24 |
Peso molecular |
1449.2 g/mol |
Nombre IUPAC |
48-[3-[(4S)-4-amino-5-hydroxy-4,6-dimethyloxan-2-yl]oxy-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-22-(2-amino-2-oxoethyl)-5,15-dichloro-2,18,32,35,37-pentahydroxy-19-[[4-methyl-2-(methylamino)pentanoyl]amino]-20,23,26,42,44-pentaoxo-7,13-dioxa-21,24,27,41,43-pentazaoctacyclo[26.14.2.23,6.214,17.18,12.129,33.010,25.034,39]pentaconta-3,5,8(48),9,11,14,16,29(45),30,32,34(39),35,37,46,49-pentadecaene-40-carboxylic acid |
InChI |
InChI=1S/C66H75Cl2N9O24/c1-23(2)12-34(71-5)58(88)76-49-51(83)26-7-10-38(32(67)14-26)97-40-16-28-17-41(55(40)101-65-56(54(86)53(85)42(22-78)99-65)100-44-21-66(4,70)57(87)24(3)96-44)98-39-11-8-27(15-33(39)68)52(84)50-63(93)75-48(64(94)95)31-18-29(79)19-37(81)45(31)30-13-25(6-9-36(30)80)46(60(90)77-50)74-61(91)47(28)73-59(89)35(20-43(69)82)72-62(49)92/h6-11,13-19,23-24,34-35,42,44,46-54,56-57,65,71,78-81,83-87H,12,20-22,70H2,1-5H3,(H2,69,82)(H,72,92)(H,73,89)(H,74,91)(H,75,93)(H,76,88)(H,77,90)(H,94,95)/t24?,34?,35?,42?,44?,46?,47?,48?,49?,50?,51?,52?,53?,54?,56?,57?,65?,66-/m0/s1 |
Clave InChI |
MYPYJXKWCTUITO-BSRCFTEOSA-N |
SMILES |
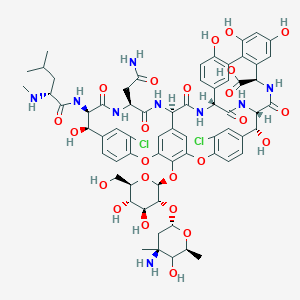
CC1C(C(CC(O1)OC2C(C(C(OC2OC3=C4C=C5C=C3OC6=C(C=C(C=C6)C(C(C(=O)NC(C(=O)NC5C(=O)NC7C8=CC(=C(C=C8)O)C9=C(C=C(C=C9O)O)C(NC(=O)C(C(C1=CC(=C(O4)C=C1)Cl)O)NC7=O)C(=O)O)CC(=O)N)NC(=O)C(CC(C)C)NC)O)Cl)CO)O)O)(C)N)O |
SMILES isomérico |
CC1C([C@@](CC(O1)OC2C(C(C(OC2OC3=C4C=C5C=C3OC6=C(C=C(C=C6)C(C(C(=O)NC(C(=O)NC5C(=O)NC7C8=CC(=C(C=C8)O)C9=C(C=C(C=C9O)O)C(NC(=O)C(C(C1=CC(=C(O4)C=C1)Cl)O)NC7=O)C(=O)O)CC(=O)N)NC(=O)C(CC(C)C)NC)O)Cl)CO)O)O)(C)N)O |
SMILES canónico |
CC1C(C(CC(O1)OC2C(C(C(OC2OC3=C4C=C5C=C3OC6=C(C=C(C=C6)C(C(C(=O)NC(C(=O)NC5C(=O)NC7C8=CC(=C(C=C8)O)C9=C(C=C(C=C9O)O)C(NC(=O)C(C(C1=CC(=C(O4)C=C1)Cl)O)NC7=O)C(=O)O)CC(=O)N)NC(=O)C(CC(C)C)NC)O)Cl)CO)O)O)(C)N)O |
Apariencia |
White to off-white solid powder |
| Vancomycin is an antibiotic used to treat a number of bacterial infections. It is a member of the glycopeptide antibiotic class and is effective mostly against Gram-positive bacteria. | |
Punto de ebullición |
N/A |
melting_point |
N/A |
| 1404-90-6 | |
Descripción física |
Tan to brown solid; [HSDB] |
Pictogramas |
Health Hazard |
Pureza |
>98% (or refer to the Certificate of Analysis) |
Números CAS relacionados |
1404-93-9 (hydrochloride) 64685-75-2 (sulfate) |
Vida útil |
When reconstituted with sterile water for injection, vancomycin hydrochloride injection is stable for 2 weeks at room temperature; the manufacturers state that reconstituted injections may be stored for 96 hours at 2 - 8 °C without substantial loss of potency. When reconstituted as directed in 0.9% sodium chloride injection or 5% dextrose injection, solutions prepared from ADD-Vantage vials of the drug are stable for 24 hours at room temperature. Vancomycin solutions containing 5 mg/mL in 0.9% sodium chloride injection or 5% dextrose injection are reportedly stable for at least 17 days when stored at 24 °C in glass or PVC containers and for at least 63 days when stored at 5 °C or -10 °C in glass containers. Following reconstitution with sterile water for injection as directed, vancomycin solutions that have been further diluted to a concentration of 5 mg/mL in 5 - 30% dextrose injection are stable when stored in plastic syringes for 24 hours at 4 eg C and then subsequently for 2 hours at room temperature. Solutions are stable for two weeks at room temp or longer if refrigerated. /Hydrochloride/ |
Solubilidad |
White solid; solubility in water: greater than 100 mg/mL; moderately soluble in methanol; insoluble in higher alcohols, acetone, ether; UV max absorption (water): 282 nm (e = 40, 1%, 1 cm) /Vancomycin hydrochloride/ |
Fuente |
Synthetic |
Sinónimos |
AB-Vancomycin Diatracin Hydrochloride, Vancomycin Sulfate, Vancomycin Vanco Azupharma VANCO-cell Vanco-saar Vancocin Vancocin HCl Vancocine Vancomicina Abbott Vancomicina Chiesi Vancomicina Combino Phar Vancomicina Norman Vancomycin Vancomycin Hexal Vancomycin Hydrochloride Vancomycin Lilly Vancomycin Phosphate (1:2) Vancomycin Phosphate (1:2), Decahydrate Vancomycin Sulfate Vancomycin-ratiopharm Vancomycine Dakota |
Origen del producto |
United States |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.