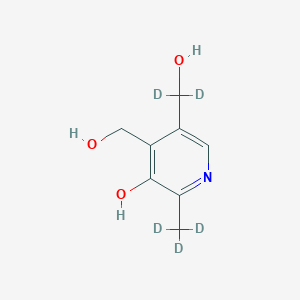
Pyridoxine-d5
Número de catálogo B025862
Peso molecular: 174.21 g/mol
Clave InChI: LXNHXLLTXMVWPM-WNWXXORZSA-N
Atención: Solo para uso de investigación. No para uso humano o veterinario.
Patent
US07371547B2
Procedure details


In a similar manner as described in Example 3, S. meliloti PY-C341K1 was cultured in a flask containing LBMCG containing 10 μg/ml of Tc for 16 hours at 30° C., and the cell suspension of the strain was prepared. A tube containing 5 ml of the reaction mixtures composed of 0, 30, and 50 μg/ml of NTG and 1.6×109 cells per ml in 50 mM Tris-HCl buffer (pH 8.0) was incubated with a reciprocal shaking (275 rpm) for 30 min at 30° C. The cells of each reaction mixture were washed twice with sterile saline and suspended in saline. 100 μl of the cell suspension was spread onto agar plates containing LBMCG containing 10 μg/ml of Tc, and then the plates were incubated for 2-3 days at 30° C. The cells grown on the plates were recovered by suspending in sterile saline. After centrifugation of the suspension, the cell suspension was diluted to give a turbidity of OD600=1.6, and finally to 10−5. Each 100 μl of the diluents was spread onto five agar plates containing LBMCG containing 10 μg/ml of Tc and 0, 0.125, 0.15, or 0.175% glycine because 0.15% glycine completely inhibited the growth of S. meliloti PY-C341K1 on LBMCG plate, and then the plates were incubated for 4 days at 30° C. Ten colonies treated with 50 μg/ml of NTG grown on plates LBMCG containing 10 μg/ml of Tc and 0.175% glycine were picked up on LBMCG agar containing 10 μg/ml of Tc. After incubation for 2 days at 30° C., the productivity of vitamin B6 in ten colonies together with the parent strain (S. meliloti PY-C341K1) was examined by flask fermentation. One loopful cells was inoculated to tubes containing 8 ml of SM medium, and then the tubes were shaken on a reciprocal shaker (275 rpm) at 30° C. After shaking for 19 hours, each 4 ml of culture broth was transferred to a 500-ml flask with two baffles containing 200 ml of PM medium modified to 0.175% NH4Cl, and shaken on a rotary shaker (180 rpm) at 30° C. After shaking for 4 days, sterile solution of urea was added to the each flask at 0.125%, and the shaking were further continued for 3 days. The contents of vitamin B6 in the supernatant of 7-day culture broth were quantified by HPLC method as described in Example 3. As a result, S. meliloti PY-EGC1 produced 362 mg of pyridoxol per liter and was about 2.11 times higher than strain PY-341K1 (the parent).
[Compound]
Name
reaction
Quantity
5 mL
Type
reactant
Reaction Step One








Name
Tris-HCl
Quantity
0 (± 1) mol
Type
solvent
Reaction Step Eight

Name
Identifiers


|
REACTION_CXSMILES
|
NCC(O)=O.[CH3:6][C:7]1[N:12]=[CH:11][C:10]([CH2:13][OH:14])=[C:9]([CH2:15][OH:16])[C:8]=1[OH:17].Cl.[NH4+].[Cl-].NC(N)=O>C(O)C(N)(CO)CO.Cl>[CH3:6][C:7]1[C:8]([OH:17])=[C:9]([CH2:15][OH:16])[C:10]([CH2:13][OH:14])=[CH:11][N:12]=1 |f:1.2,3.4,6.7|
|
Inputs


Step One
[Compound]
|
Name
|
reaction
|
|
Quantity
|
5 mL
|
|
Type
|
reactant
|
|
Smiles
|
|
Step Two
Step Three
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
NCC(=O)O
|
Step Four
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
CC1=C(C(=C(C=N1)CO)CO)O.Cl
|
Step Five
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
[NH4+].[Cl-]
|
Step Six
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
NC(=O)N
|
Step Seven
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
CC1=C(C(=C(C=N1)CO)CO)O.Cl
|
Step Eight
|
Name
|
Tris-HCl
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
solvent
|
|
Smiles
|
C(C(CO)(CO)N)O.Cl
|
Conditions


Temperature
|
Control Type
|
UNSPECIFIED
|
|
Setpoint
|
30 °C
|
Stirring
|
Type
|
CUSTOM
|
|
Details
|
a reciprocal shaking (275 rpm) for 30 min at 30° C
|
|
Rate
|
UNSPECIFIED
|
|
RPM
|
0
|
Other
|
Conditions are dynamic
|
1
|
|
Details
|
See reaction.notes.procedure_details.
|
Workups


CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
was cultured in a flask
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
containing LBMCG
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
containing 10 μg/ml of Tc for 16 hours at 30° C.
|
|
Duration
|
16 h
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
the cell suspension of the strain
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
was prepared
|
WASH
|
Type
|
WASH
|
|
Details
|
The cells of each reaction mixture were washed twice with sterile saline
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
containing LBMCG
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
containing 10 μg/ml of Tc
|
WAIT
|
Type
|
WAIT
|
|
Details
|
the plates were incubated for 2-3 days at 30° C
|
|
Duration
|
2.5 (± 0.5) d
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
were recovered
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
After centrifugation of the suspension, the cell suspension was diluted
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
to give a turbidity of OD600=1.6
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
containing LBMCG
|
WAIT
|
Type
|
WAIT
|
|
Details
|
on LBMCG plate, and then the plates were incubated for 4 days at 30° C
|
|
Duration
|
4 d
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
Ten colonies treated with 50 μg/ml of NTG grown on plates LBMCG
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
containing 10 μg/ml of Tc
|
WAIT
|
Type
|
WAIT
|
|
Details
|
After incubation for 2 days at 30° C.
|
|
Duration
|
2 d
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
containing 8 ml of SM medium
|
STIRRING
|
Type
|
STIRRING
|
|
Details
|
the tubes were shaken on a reciprocal shaker (275 rpm) at 30° C
|
STIRRING
|
Type
|
STIRRING
|
|
Details
|
After shaking for 19 hours
|
|
Duration
|
19 h
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
each 4 ml of culture broth was transferred to a 500-ml flask with two baffles
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
containing 200 ml of PM medium
|
STIRRING
|
Type
|
STIRRING
|
|
Details
|
shaken on a rotary shaker (180 rpm) at 30° C
|
STIRRING
|
Type
|
STIRRING
|
|
Details
|
After shaking for 4 days
|
|
Duration
|
4 d
|
WAIT
|
Type
|
WAIT
|
|
Details
|
the shaking were further continued for 3 days
|
|
Duration
|
3 d
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
in the supernatant of 7-day culture broth
|
Outcomes


Product
Details
Reaction Time |
30 min |
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
CC1=NC=C(C(=C1O)CO)CO
|
Measurements
| Type | Value | Analysis |
|---|---|---|
| AMOUNT: MASS | 362 mg |
Source


|
Source
|
Open Reaction Database (ORD) |
|
Description
|
The Open Reaction Database (ORD) is an open-access schema and infrastructure for structuring and sharing organic reaction data, including a centralized data repository. The ORD schema supports conventional and emerging technologies, from benchtop reactions to automated high-throughput experiments and flow chemistry. Our vision is that a consistent data representation and infrastructure to support data sharing will enable downstream applications that will greatly improve the state of the art with respect to computer-aided synthesis planning, reaction prediction, and other predictive chemistry tasks. |
Patent
US07371547B2
Procedure details


In a similar manner as described in Example 3, S. meliloti PY-C341K1 was cultured in a flask containing LBMCG containing 10 μg/ml of Tc for 16 hours at 30° C., and the cell suspension of the strain was prepared. A tube containing 5 ml of the reaction mixtures composed of 0, 30, and 50 μg/ml of NTG and 1.6×109 cells per ml in 50 mM Tris-HCl buffer (pH 8.0) was incubated with a reciprocal shaking (275 rpm) for 30 min at 30° C. The cells of each reaction mixture were washed twice with sterile saline and suspended in saline. 100 μl of the cell suspension was spread onto agar plates containing LBMCG containing 10 μg/ml of Tc, and then the plates were incubated for 2-3 days at 30° C. The cells grown on the plates were recovered by suspending in sterile saline. After centrifugation of the suspension, the cell suspension was diluted to give a turbidity of OD600=1.6, and finally to 10−5. Each 100 μl of the diluents was spread onto five agar plates containing LBMCG containing 10 μg/ml of Tc and 0, 0.125, 0.15, or 0.175% glycine because 0.15% glycine completely inhibited the growth of S. meliloti PY-C341K1 on LBMCG plate, and then the plates were incubated for 4 days at 30° C. Ten colonies treated with 50 μg/ml of NTG grown on plates LBMCG containing 10 μg/ml of Tc and 0.175% glycine were picked up on LBMCG agar containing 10 μg/ml of Tc. After incubation for 2 days at 30° C., the productivity of vitamin B6 in ten colonies together with the parent strain (S. meliloti PY-C341K1) was examined by flask fermentation. One loopful cells was inoculated to tubes containing 8 ml of SM medium, and then the tubes were shaken on a reciprocal shaker (275 rpm) at 30° C. After shaking for 19 hours, each 4 ml of culture broth was transferred to a 500-ml flask with two baffles containing 200 ml of PM medium modified to 0.175% NH4Cl, and shaken on a rotary shaker (180 rpm) at 30° C. After shaking for 4 days, sterile solution of urea was added to the each flask at 0.125%, and the shaking were further continued for 3 days. The contents of vitamin B6 in the supernatant of 7-day culture broth were quantified by HPLC method as described in Example 3. As a result, S. meliloti PY-EGC1 produced 362 mg of pyridoxol per liter and was about 2.11 times higher than strain PY-341K1 (the parent).
[Compound]
Name
reaction
Quantity
5 mL
Type
reactant
Reaction Step One








Name
Tris-HCl
Quantity
0 (± 1) mol
Type
solvent
Reaction Step Eight

Name
Identifiers


|
REACTION_CXSMILES
|
NCC(O)=O.[CH3:6][C:7]1[N:12]=[CH:11][C:10]([CH2:13][OH:14])=[C:9]([CH2:15][OH:16])[C:8]=1[OH:17].Cl.[NH4+].[Cl-].NC(N)=O>C(O)C(N)(CO)CO.Cl>[CH3:6][C:7]1[C:8]([OH:17])=[C:9]([CH2:15][OH:16])[C:10]([CH2:13][OH:14])=[CH:11][N:12]=1 |f:1.2,3.4,6.7|
|
Inputs


Step One
[Compound]
|
Name
|
reaction
|
|
Quantity
|
5 mL
|
|
Type
|
reactant
|
|
Smiles
|
|
Step Two
Step Three
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
NCC(=O)O
|
Step Four
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
CC1=C(C(=C(C=N1)CO)CO)O.Cl
|
Step Five
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
[NH4+].[Cl-]
|
Step Six
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
NC(=O)N
|
Step Seven
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
CC1=C(C(=C(C=N1)CO)CO)O.Cl
|
Step Eight
|
Name
|
Tris-HCl
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
solvent
|
|
Smiles
|
C(C(CO)(CO)N)O.Cl
|
Conditions


Temperature
|
Control Type
|
UNSPECIFIED
|
|
Setpoint
|
30 °C
|
Stirring
|
Type
|
CUSTOM
|
|
Details
|
a reciprocal shaking (275 rpm) for 30 min at 30° C
|
|
Rate
|
UNSPECIFIED
|
|
RPM
|
0
|
Other
|
Conditions are dynamic
|
1
|
|
Details
|
See reaction.notes.procedure_details.
|
Workups


CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
was cultured in a flask
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
containing LBMCG
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
containing 10 μg/ml of Tc for 16 hours at 30° C.
|
|
Duration
|
16 h
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
the cell suspension of the strain
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
was prepared
|
WASH
|
Type
|
WASH
|
|
Details
|
The cells of each reaction mixture were washed twice with sterile saline
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
containing LBMCG
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
containing 10 μg/ml of Tc
|
WAIT
|
Type
|
WAIT
|
|
Details
|
the plates were incubated for 2-3 days at 30° C
|
|
Duration
|
2.5 (± 0.5) d
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
were recovered
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
After centrifugation of the suspension, the cell suspension was diluted
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
to give a turbidity of OD600=1.6
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
containing LBMCG
|
WAIT
|
Type
|
WAIT
|
|
Details
|
on LBMCG plate, and then the plates were incubated for 4 days at 30° C
|
|
Duration
|
4 d
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
Ten colonies treated with 50 μg/ml of NTG grown on plates LBMCG
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
containing 10 μg/ml of Tc
|
WAIT
|
Type
|
WAIT
|
|
Details
|
After incubation for 2 days at 30° C.
|
|
Duration
|
2 d
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
containing 8 ml of SM medium
|
STIRRING
|
Type
|
STIRRING
|
|
Details
|
the tubes were shaken on a reciprocal shaker (275 rpm) at 30° C
|
STIRRING
|
Type
|
STIRRING
|
|
Details
|
After shaking for 19 hours
|
|
Duration
|
19 h
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
each 4 ml of culture broth was transferred to a 500-ml flask with two baffles
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
containing 200 ml of PM medium
|
STIRRING
|
Type
|
STIRRING
|
|
Details
|
shaken on a rotary shaker (180 rpm) at 30° C
|
STIRRING
|
Type
|
STIRRING
|
|
Details
|
After shaking for 4 days
|
|
Duration
|
4 d
|
WAIT
|
Type
|
WAIT
|
|
Details
|
the shaking were further continued for 3 days
|
|
Duration
|
3 d
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
in the supernatant of 7-day culture broth
|
Outcomes


Product
Details
Reaction Time |
30 min |
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
CC1=NC=C(C(=C1O)CO)CO
|
Measurements
| Type | Value | Analysis |
|---|---|---|
| AMOUNT: MASS | 362 mg |
Source


|
Source
|
Open Reaction Database (ORD) |
|
Description
|
The Open Reaction Database (ORD) is an open-access schema and infrastructure for structuring and sharing organic reaction data, including a centralized data repository. The ORD schema supports conventional and emerging technologies, from benchtop reactions to automated high-throughput experiments and flow chemistry. Our vision is that a consistent data representation and infrastructure to support data sharing will enable downstream applications that will greatly improve the state of the art with respect to computer-aided synthesis planning, reaction prediction, and other predictive chemistry tasks. |
Patent
US07371547B2
Procedure details


In a similar manner as described in Example 3, S. meliloti PY-C341K1 was cultured in a flask containing LBMCG containing 10 μg/ml of Tc for 16 hours at 30° C., and the cell suspension of the strain was prepared. A tube containing 5 ml of the reaction mixtures composed of 0, 30, and 50 μg/ml of NTG and 1.6×109 cells per ml in 50 mM Tris-HCl buffer (pH 8.0) was incubated with a reciprocal shaking (275 rpm) for 30 min at 30° C. The cells of each reaction mixture were washed twice with sterile saline and suspended in saline. 100 μl of the cell suspension was spread onto agar plates containing LBMCG containing 10 μg/ml of Tc, and then the plates were incubated for 2-3 days at 30° C. The cells grown on the plates were recovered by suspending in sterile saline. After centrifugation of the suspension, the cell suspension was diluted to give a turbidity of OD600=1.6, and finally to 10−5. Each 100 μl of the diluents was spread onto five agar plates containing LBMCG containing 10 μg/ml of Tc and 0, 0.125, 0.15, or 0.175% glycine because 0.15% glycine completely inhibited the growth of S. meliloti PY-C341K1 on LBMCG plate, and then the plates were incubated for 4 days at 30° C. Ten colonies treated with 50 μg/ml of NTG grown on plates LBMCG containing 10 μg/ml of Tc and 0.175% glycine were picked up on LBMCG agar containing 10 μg/ml of Tc. After incubation for 2 days at 30° C., the productivity of vitamin B6 in ten colonies together with the parent strain (S. meliloti PY-C341K1) was examined by flask fermentation. One loopful cells was inoculated to tubes containing 8 ml of SM medium, and then the tubes were shaken on a reciprocal shaker (275 rpm) at 30° C. After shaking for 19 hours, each 4 ml of culture broth was transferred to a 500-ml flask with two baffles containing 200 ml of PM medium modified to 0.175% NH4Cl, and shaken on a rotary shaker (180 rpm) at 30° C. After shaking for 4 days, sterile solution of urea was added to the each flask at 0.125%, and the shaking were further continued for 3 days. The contents of vitamin B6 in the supernatant of 7-day culture broth were quantified by HPLC method as described in Example 3. As a result, S. meliloti PY-EGC1 produced 362 mg of pyridoxol per liter and was about 2.11 times higher than strain PY-341K1 (the parent).
[Compound]
Name
reaction
Quantity
5 mL
Type
reactant
Reaction Step One








Name
Tris-HCl
Quantity
0 (± 1) mol
Type
solvent
Reaction Step Eight

Name
Identifiers


|
REACTION_CXSMILES
|
NCC(O)=O.[CH3:6][C:7]1[N:12]=[CH:11][C:10]([CH2:13][OH:14])=[C:9]([CH2:15][OH:16])[C:8]=1[OH:17].Cl.[NH4+].[Cl-].NC(N)=O>C(O)C(N)(CO)CO.Cl>[CH3:6][C:7]1[C:8]([OH:17])=[C:9]([CH2:15][OH:16])[C:10]([CH2:13][OH:14])=[CH:11][N:12]=1 |f:1.2,3.4,6.7|
|
Inputs


Step One
[Compound]
|
Name
|
reaction
|
|
Quantity
|
5 mL
|
|
Type
|
reactant
|
|
Smiles
|
|
Step Two
Step Three
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
NCC(=O)O
|
Step Four
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
CC1=C(C(=C(C=N1)CO)CO)O.Cl
|
Step Five
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
[NH4+].[Cl-]
|
Step Six
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
NC(=O)N
|
Step Seven
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
CC1=C(C(=C(C=N1)CO)CO)O.Cl
|
Step Eight
|
Name
|
Tris-HCl
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
solvent
|
|
Smiles
|
C(C(CO)(CO)N)O.Cl
|
Conditions


Temperature
|
Control Type
|
UNSPECIFIED
|
|
Setpoint
|
30 °C
|
Stirring
|
Type
|
CUSTOM
|
|
Details
|
a reciprocal shaking (275 rpm) for 30 min at 30° C
|
|
Rate
|
UNSPECIFIED
|
|
RPM
|
0
|
Other
|
Conditions are dynamic
|
1
|
|
Details
|
See reaction.notes.procedure_details.
|
Workups


CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
was cultured in a flask
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
containing LBMCG
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
containing 10 μg/ml of Tc for 16 hours at 30° C.
|
|
Duration
|
16 h
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
the cell suspension of the strain
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
was prepared
|
WASH
|
Type
|
WASH
|
|
Details
|
The cells of each reaction mixture were washed twice with sterile saline
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
containing LBMCG
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
containing 10 μg/ml of Tc
|
WAIT
|
Type
|
WAIT
|
|
Details
|
the plates were incubated for 2-3 days at 30° C
|
|
Duration
|
2.5 (± 0.5) d
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
were recovered
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
After centrifugation of the suspension, the cell suspension was diluted
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
to give a turbidity of OD600=1.6
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
containing LBMCG
|
WAIT
|
Type
|
WAIT
|
|
Details
|
on LBMCG plate, and then the plates were incubated for 4 days at 30° C
|
|
Duration
|
4 d
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
Ten colonies treated with 50 μg/ml of NTG grown on plates LBMCG
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
containing 10 μg/ml of Tc
|
WAIT
|
Type
|
WAIT
|
|
Details
|
After incubation for 2 days at 30° C.
|
|
Duration
|
2 d
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
containing 8 ml of SM medium
|
STIRRING
|
Type
|
STIRRING
|
|
Details
|
the tubes were shaken on a reciprocal shaker (275 rpm) at 30° C
|
STIRRING
|
Type
|
STIRRING
|
|
Details
|
After shaking for 19 hours
|
|
Duration
|
19 h
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
each 4 ml of culture broth was transferred to a 500-ml flask with two baffles
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
containing 200 ml of PM medium
|
STIRRING
|
Type
|
STIRRING
|
|
Details
|
shaken on a rotary shaker (180 rpm) at 30° C
|
STIRRING
|
Type
|
STIRRING
|
|
Details
|
After shaking for 4 days
|
|
Duration
|
4 d
|
WAIT
|
Type
|
WAIT
|
|
Details
|
the shaking were further continued for 3 days
|
|
Duration
|
3 d
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
in the supernatant of 7-day culture broth
|
Outcomes


Product
Details
Reaction Time |
30 min |
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
CC1=NC=C(C(=C1O)CO)CO
|
Measurements
| Type | Value | Analysis |
|---|---|---|
| AMOUNT: MASS | 362 mg |
Source


|
Source
|
Open Reaction Database (ORD) |
|
Description
|
The Open Reaction Database (ORD) is an open-access schema and infrastructure for structuring and sharing organic reaction data, including a centralized data repository. The ORD schema supports conventional and emerging technologies, from benchtop reactions to automated high-throughput experiments and flow chemistry. Our vision is that a consistent data representation and infrastructure to support data sharing will enable downstream applications that will greatly improve the state of the art with respect to computer-aided synthesis planning, reaction prediction, and other predictive chemistry tasks. |
