Diéthylcarbamazine
Vue d'ensemble
Description
La diéthylcarbamazine est un composé organique synthétique principalement utilisé comme médicament anthelminthique. Elle est efficace contre certains vers filaires parasites, qui sont endémiques dans la plupart des régions subtropicales et tropicales du monde. Ces parasites infectent le sang et les canaux lymphatiques chez l’homme, causant la filariose, une maladie débilitante . La this compound a été découverte en 1947 par Yellapragada Subbarow et figure sur la Liste des médicaments essentiels de l’Organisation mondiale de la santé .
Applications De Recherche Scientifique
Diethylcarbamazine has a wide range of scientific research applications, including:
Chemistry: It is used as a reagent in organic synthesis to study various chemical reactions and mechanisms.
Medicine: It is primarily used in the treatment of filariasis, including lymphatic filariasis, tropical pulmonary eosinophilia, and loiasis.
Industry: Diethylcarbamazine is used in the pharmaceutical industry for the production of anthelmintic drugs.
Mécanisme D'action
La diéthylcarbamazine exerce ses effets en inhibant le métabolisme de l’acide arachidonique dans les microfilaires. Cette inhibition rend les microfilaires plus sensibles à l’attaque immunitaire innée, mais ne tue pas les parasites directement . Le composé sensibilise les microfilaires à la phagocytose, et son activité dépend de la synthase inductible de l’oxyde nitrique et de la voie de la cyclooxygénase . De plus, la this compound cible la voie de la cyclooxygénase et la COX-1 .
Analyse Biochimique
Biochemical Properties
Diethylcarbamazine plays a crucial role in biochemical reactions by interacting with various enzymes, proteins, and other biomolecules. One of its primary mechanisms involves the inhibition of arachidonic acid metabolism in microfilariae, making them more susceptible to the host’s immune response . Diethylcarbamazine interacts with inducible nitric-oxide synthase and the cyclooxygenase pathway, which are essential for its antiparasitic activity .
Cellular Effects
Diethylcarbamazine affects various types of cells and cellular processes. It influences cell function by altering cell signaling pathways, gene expression, and cellular metabolism. The compound has been shown to immobilize the muscles of parasitic worms and alter their skin membranes, enhancing the host immune system’s ability to kill the worms . Additionally, diethylcarbamazine’s effects on the arachidonic acid metabolic pathway play a significant role in its cellular impact .
Molecular Mechanism
The molecular mechanism of diethylcarbamazine involves sensitizing microfilariae to phagocytosis. This process is dependent on inducible nitric-oxide synthase and the cyclooxygenase pathway . Diethylcarbamazine interferes with the arachidonic acid metabolism of the parasite, causing the death of the microfilariae . It also affects the production of thromboxane, prostacyclin, prostaglandin, and leukotrienes by interfering with the cyclooxygenase and lipoxygenase pathways .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of diethylcarbamazine change over time. The compound is known for its stability and effectiveness in treating filarial infections. Its long-term effects on cellular function have been observed in both in vitro and in vivo studies. Diethylcarbamazine’s activity against Brugia malayi microfilariae is dependent on the inducible nitric-oxide synthase and the cyclooxygenase pathway . The compound’s stability and degradation over time are crucial factors in its effectiveness.
Dosage Effects in Animal Models
The effects of diethylcarbamazine vary with different dosages in animal models. Studies have shown that higher doses of diethylcarbamazine can lead to toxic or adverse effects. For example, the oral LD50 in rats and mice is 1400 mg/kg and 660 mg/kg, respectively . In gerbils infected with Brugia malayi, diethylcarbamazine treatment resulted in significant changes in gene expression, indicating its impact on parasite survival, development, and reproduction .
Metabolic Pathways
Diethylcarbamazine is involved in several metabolic pathways, including the arachidonic acid metabolic pathway. It targets the cyclooxygenase pathway and COX-1, which are essential for its antiparasitic activity . The compound’s interaction with these pathways leads to the production of metabolites that are primarily excreted through renal clearance .
Transport and Distribution
Diethylcarbamazine is widely distributed throughout all body compartments, except adipose tissue . It is rapidly absorbed after oral administration, with peak serum levels occurring in about 3 hours . The compound is primarily excreted in the urine as metabolites or unchanged drug .
Subcellular Localization
The subcellular localization of diethylcarbamazine is crucial for its activity and function. The compound’s interaction with specific cellular compartments and organelles is essential for its antiparasitic effects. Diethylcarbamazine’s targeting signals and post-translational modifications direct it to specific compartments, enhancing its effectiveness against parasitic infections .
Méthodes De Préparation
Voies de Synthèse et Conditions de Réaction : La diéthylcarbamazine est synthétisée à partir de la 4-méthylpipérazine. La synthèse implique la réaction de la 4-méthylpipérazine avec le chlorure de diéthylcarbamoyle dans des conditions contrôlées pour former la this compound .
Méthodes de Production Industrielle : En milieu industriel, la this compound est produite par réaction de la 4-méthylpipérazine avec le chlorure de diéthylcarbamoyle en présence d’un solvant et d’un catalyseur appropriés. La réaction est généralement effectuée à des températures élevées pour assurer une conversion complète des réactifs en produit souhaité .
Analyse Des Réactions Chimiques
Types de Réactions : La diéthylcarbamazine subit diverses réactions chimiques, notamment :
Oxydation : La this compound peut être oxydée pour former de la this compound-N-oxyde.
Réduction : Les réactions de réduction peuvent convertir la this compound en ses dérivés aminés correspondants.
Substitution : La this compound peut subir des réactions de substitution avec divers électrophiles pour former des dérivés substitués.
Réactifs et Conditions Communs :
Principaux Produits Formés :
Oxydation : this compound-N-oxyde.
Réduction : Dérivés aminés de la this compound.
Substitution : Dérivés substitués de la this compound.
4. Applications de la Recherche Scientifique
La this compound a un large éventail d’applications de recherche scientifique, notamment :
Comparaison Avec Des Composés Similaires
La diéthylcarbamazine est unique parmi les médicaments anthelminthiques en raison de son mécanisme d’action spécifique et de son efficacité contre un large éventail de parasites filaires . Des composés similaires comprennent :
Ivermectine : Préférée pour le traitement de l’onchocercose (cécité des rivières), mais moins efficace contre d’autres infections filaires.
Levamisole : Un autre médicament anthelminthique avec un mécanisme d’action différent, principalement utilisé en médecine vétérinaire.
La this compound se distingue par son ciblage spécifique du métabolisme de l’acide arachidonique et par son activité à large spectre contre divers parasites filaires .
Propriétés
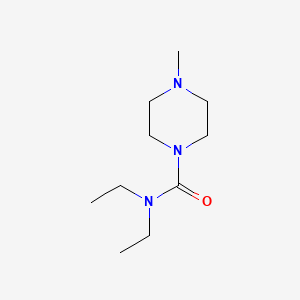
IUPAC Name |
N,N-diethyl-4-methylpiperazine-1-carboxamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C10H21N3O/c1-4-12(5-2)10(14)13-8-6-11(3)7-9-13/h4-9H2,1-3H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
RCKMWOKWVGPNJF-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCN(CC)C(=O)N1CCN(CC1)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C10H21N3O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
1642-54-2 (citrate (1:1)), 5348-97-0 (mono-hydrochloride) | |
| Record name | Diethylcarbamazine [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000090891 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID1022928 | |
| Record name | Diethylcarbamazine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID1022928 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
199.29 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Diethylcarbamazine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014849 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
2.36e+02 g/L | |
| Record name | Diethylcarbamazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00711 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Diethylcarbamazine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014849 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
The mechanism of action of diethylcarbamazine is thought to involve sensitizing the microfilariae to phagocytosis. One study showed that diethylcarbamazine's activity against Brugia malayi microfilariae is dependent on inducible nitric-oxide synthase and the cyclooxygenase pathway. It confirmed the important role of the arachidonic acid metabolic pathway in diethylcarbamazine's mechanism of action in vivo and showes that in addition to its effects on the 5-lipoxygenase pathway, it targets the cyclooxygenase pathway and COX-1. | |
| Record name | Diethylcarbamazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00711 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
90-89-1 | |
| Record name | Diethylcarbamazine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=90-89-1 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Diethylcarbamazine [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000090891 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Diethylcarbamazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00711 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | 1-Piperazinecarboxamide, N,N-diethyl-4-methyl- | |
| Source | EPA Chemicals under the TSCA | |
| URL | https://www.epa.gov/chemicals-under-tsca | |
| Description | EPA Chemicals under the Toxic Substances Control Act (TSCA) collection contains information on chemicals and their regulations under TSCA, including non-confidential content from the TSCA Chemical Substance Inventory and Chemical Data Reporting. | |
| Record name | Diethylcarbamazine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID1022928 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Diethylcarbamazine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.001.840 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | DIETHYLCARBAMAZINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/V867Q8X3ZD | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Diethylcarbamazine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014849 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
150-155, 48 °C | |
| Record name | Diethylcarbamazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00711 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Diethylcarbamazine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014849 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: How does DEC affect calcium signaling in Brugia malayi muscles?
A1: DEC interacts with the transient receptor potential channel, C classification (TRPC) ortholog receptor TRP-2 in Brugia malayi muscles. This interaction promotes calcium ion (Ca2+) entry into the muscle cells, leading to muscle contraction and potentially contributing to paralysis. []
Q2: Does DEC have any synergistic effects with other anthelmintics?
A2: Yes, DEC demonstrates synergistic effects with emodepside, an anthelmintic drug. Emodepside is thought to target Slopoke (SLO-1) Ca2+-activated potassium (K+) channels. When combined with DEC, emodepside potentiates the DEC-induced Ca2+ signal in Brugia malayi muscles, leading to enhanced inhibition of motility. []
Q3: What is the molecular formula and weight of diethylcarbamazine?
A3: Diethylcarbamazine has the molecular formula C10H21N3O and a molecular weight of 199.29 g/mol.
Q4: Does the packaging material influence the stability of DEC tablets?
A4: Yes, packaging plays a crucial role in maintaining DEC stability. A study indicated that aluminum strips with polyethylene lining are the most appropriate packaging material to ensure the stability of DEC 50 mg tablets throughout their shelf life. []
Q5: How is DEC absorbed and distributed in the body?
A5: DEC is rapidly absorbed following oral administration. [] It is then widely distributed throughout the body, including saliva. Studies have shown a strong correlation between DEC concentrations in plasma and saliva, suggesting that saliva concentrations could be used to monitor drug therapy. []
Q6: How is DEC eliminated from the body?
A6: DEC is primarily metabolized in the liver and excreted in the urine. Activated charcoal has been shown to reduce the absorption and urinary excretion of DEC by adsorbing it in the gastrointestinal tract. []
Q7: What is the efficacy of combination therapy with DEC?
A7: Combination therapies involving DEC often demonstrate superior efficacy compared to single-drug treatments. For instance, a combination of DEC and albendazole was found to be more effective at clearing Brugia malayi microfilariae than either drug alone. [] Similarly, annual mass treatment with DEC plus ivermectin resulted in a greater reduction in microfilariae-positive infections compared to DEC alone in a study conducted in Papua New Guinea. []
Q8: Is there evidence of resistance to DEC in filarial parasites?
A8: While widespread resistance to DEC has not been widely reported, the potential for its development exists. Continuous monitoring for resistance is crucial to ensure the long-term effectiveness of DEC-based treatment strategies.
Q9: What are some common adverse reactions associated with DEC treatment?
A9: DEC treatment can cause adverse reactions, particularly in individuals infected with filarial parasites. These reactions, often termed Mazzotti reactions, can manifest as fever, headache, dizziness, skin reactions, and gastrointestinal complications. [, , , , ] The severity of these reactions can vary depending on the filarial species involved, with infections of Onchocerca volvulus often associated with more severe reactions. []
Q10: Can DEC administration lead to changes in blood parameters?
A10: Yes, DEC administration has been associated with a profound decrease in circulating eosinophils, reaching their lowest levels just before or during the onset of clinical and physiological changes associated with the Mazzotti reaction. []
Q11: Are there any novel drug delivery systems being explored for DEC?
A11: Researchers are exploring the development of orodispersible tablets of DEC to enhance patient compliance, particularly among pediatric and geriatric populations who may have difficulty swallowing conventional tablets. [, ]
Q12: What analytical techniques are commonly employed for the quantification of DEC?
A12: Several analytical methods are utilized for quantifying DEC, including spectrophotometry [, ] and high-performance liquid chromatography (HPLC). [, , ] These methods are essential for quality control and assurance during drug development, manufacturing, and distribution.
Q13: Have analytical methods for DEC been validated?
A13: Yes, validated RP-HPLC methods have been developed for the simultaneous estimation of DEC with other drugs like cetirizine hydrochloride and chlorpheniramine maleate in pharmaceutical formulations. These methods demonstrate good accuracy, precision, and linearity, making them suitable for routine analysis. [, ]
Q14: When was DEC first discovered as an antifilarial agent?
A14: DEC was first identified as an effective antifilarial agent in 1947. [] Its introduction marked a significant milestone in the fight against filariasis, offering a valuable tool for controlling these debilitating parasitic infections.
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.