Énalapril
Vue d'ensemble
Description
Enalapril is a medication primarily used to treat high blood pressure, heart failure, and diabetic kidney disease. It belongs to the class of drugs known as angiotensin-converting enzyme inhibitors. Enalapril is a prodrug, which means it is converted into its active form, enalaprilat, in the body. This compound works by relaxing blood vessels, thereby lowering blood pressure and improving blood flow.
Mécanisme D'action
Target of Action
Enalapril is a prodrug that belongs to the angiotensin-converting enzyme (ACE) inhibitor drug class . Its primary target is the ACE, which plays a crucial role in the renin-angiotensin-aldosterone system (RAAS), responsible for the regulation of blood pressure and fluid and electrolyte homeostasis .
Mode of Action
Enalapril is rapidly biotransformed into its active metabolite, enalaprilat, in the body . Enalaprilat competitively inhibits the ACE, hindering the production of angiotensin II, a key component of the RAAS that promotes vasoconstriction and renal reabsorption of sodium ions in the kidneys . This inhibition leads to a decrease in blood pressure and blood fluid volume .
Biochemical Pathways
The RAAS is a signaling pathway that works in synergy with the sympathetic system to regulate blood pressure and fluid and electrolyte homeostasis . By inhibiting the ACE, enalapril disrupts this pathway, leading to a decrease in the production of angiotensin II and a subsequent reduction in vasoconstriction and renal reabsorption of sodium ions .
Pharmacokinetics
Enalapril exhibits good oral absorption (60 to 70%), a rapid peak plasma concentration (1 hour), and rapid clearance (undetectable by 4 hours) by de-esterification in the liver to enalaprilat . The elimination half-life of enalaprilat is approximately 35 hours . About 61% of the administered dose is excreted in the urine, with 18% as enalapril and 43% as enalaprilat .
Result of Action
The inhibition of ACE by enalapril leads to a decrease in blood pressure and blood fluid volume . This results in the lowering of blood pressure in all grades of essential and renovascular hypertension, and peripheral vascular resistance without causing an increase in heart rate . It also exhibits natriuretic and uricosuric properties .
Action Environment
The efficacy and stability of enalapril can be influenced by various environmental factors. For instance, in individuals with renal impairment, particularly those with a glomerular filtration rate (GFR) of 30 mL/minute or less, the peak and trough enalaprilat levels increase, and time to steady state may be delayed . This necessitates dosage adjustment in such individuals .
Applications De Recherche Scientifique
Enalapril has a wide range of scientific research applications:
Chemistry: Enalapril is used as a model compound in studies involving angiotensin-converting enzyme inhibitors. Researchers study its chemical properties and reactions to develop new and improved drugs.
Biology: Enalapril is used in biological studies to understand its effects on blood pressure regulation and cardiovascular health. It is also used to study the mechanisms of action of angiotensin-converting enzyme inhibitors.
Medicine: Enalapril is widely used in clinical research to evaluate its efficacy and safety in treating various medical conditions, including hypertension, heart failure, and diabetic kidney disease. It is also used in pharmacokinetic and pharmacodynamic studies.
Industry: Enalapril is used in the pharmaceutical industry for the development and production of antihypertensive medications.
Analyse Biochimique
Biochemical Properties
Enalapril interacts with the angiotensin-converting enzyme (ACE) in the renin-angiotensin-aldosterone system . It is rapidly biotransformed into its active metabolite, enalaprilat, which competitively inhibits the ACE to hinder the production of angiotensin II . Angiotensin II is a key component of the renin-angiotensin-aldosterone system that promotes vasoconstriction and renal reabsorption of sodium ions in the kidneys .
Cellular Effects
Enalapril has several effects on cells. It reduces blood pressure and blood fluid volume . It also has side effects such as dizziness, sleepiness, and a pounding heartbeat . In some cases, it can cause serious side effects like angioedema and low blood pressure .
Molecular Mechanism
Enalapril’s active metabolite, enalaprilat, competitively inhibits the ACE . This inhibition hinders the production of angiotensin II, leading to less vasoconstriction and decreased blood pressure . This mechanism of action is how enalapril exerts its effects at the molecular level.
Temporal Effects in Laboratory Settings
Enalapril starts to reduce high blood pressure within a few hours, but it may take a few weeks to fully take effect .
Dosage Effects in Animal Models
The recommended dosage of enalapril for treatment of CHF in dogs is 0.25–0.5 mg/kg, taken orally every 12–24 hours . Based on the half-life, if continuous ACE inhibition is desired and well tolerated, then a 12-hour dosing interval is recommended .
Metabolic Pathways
Enalapril is a prodrug that is rapidly biotransformed into its active metabolite, enalaprilat . This transformation occurs in the liver . Enalaprilat then inhibits the ACE, which is part of the renin-angiotensin-aldosterone system .
Transport and Distribution
Enalapril is taken orally and is absorbed into the body where it is biotransformed into enalaprilat in the liver . It is then distributed throughout the body where it inhibits the ACE .
Méthodes De Préparation
Synthetic Routes and Reaction Conditions: Enalapril is synthesized through a multi-step process. The synthesis begins with the preparation of the key intermediate, L-alanyl-L-proline. This intermediate is then coupled with a phenylbutyric acid derivative to form enalapril. The reaction conditions typically involve the use of organic solvents and catalysts to facilitate the coupling reaction.
Industrial Production Methods: In industrial settings, enalapril is produced by reacting L-alanyl-L-proline with a phenylbutyric acid derivative under controlled conditions. The reaction mixture is then purified through crystallization and filtration to obtain the final product. The industrial process ensures high yield and purity of enalapril, making it suitable for pharmaceutical use .
Analyse Des Réactions Chimiques
Types of Reactions: Enalapril undergoes several types of chemical reactions, including hydrolysis, oxidation, and substitution.
Common Reagents and Conditions:
Hydrolysis: Enalapril is hydrolyzed in the liver to form its active metabolite, enalaprilat. This reaction is catalyzed by liver enzymes.
Oxidation: Enalapril can undergo oxidation reactions in the presence of oxidizing agents, leading to the formation of various oxidation products.
Substitution: Enalapril can participate in substitution reactions with nucleophiles, resulting in the formation of substituted derivatives.
Major Products Formed: The major product formed from the hydrolysis of enalapril is enalaprilat, which is the active form of the drug. Oxidation and substitution reactions can lead to the formation of various by-products, depending on the reagents and conditions used .
Comparaison Avec Des Composés Similaires
Lisinopril: Similar to enalapril, lisinopril is used to treat high blood pressure and heart failure. lisinopril is not a prodrug and is active in its administered form.
Ramipril: Ramipril is another angiotensin-converting enzyme inhibitor with similar uses. It is also a prodrug, converted to its active form, ramiprilat, in the body.
Captopril: Captopril is an older angiotensin-converting enzyme inhibitor that is also used to treat hypertension and heart failure. Unlike enalapril, captopril contains a sulfhydryl group, which can lead to different side effects and interactions.
Uniqueness of Enalapril: Enalapril is unique in its pharmacokinetic properties, including its conversion to the active metabolite enalaprilat. This conversion allows for a longer duration of action compared to some other angiotensin-converting enzyme inhibitors. Additionally, enalapril has been shown to have a favorable safety profile and is well-tolerated by most patients .
Propriétés
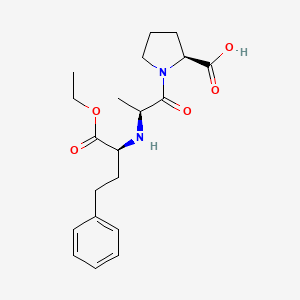
IUPAC Name |
(2S)-1-[(2S)-2-[[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino]propanoyl]pyrrolidine-2-carboxylic acid | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C20H28N2O5/c1-3-27-20(26)16(12-11-15-8-5-4-6-9-15)21-14(2)18(23)22-13-7-10-17(22)19(24)25/h4-6,8-9,14,16-17,21H,3,7,10-13H2,1-2H3,(H,24,25)/t14-,16-,17-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
GBXSMTUPTTWBMN-XIRDDKMYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCOC(=O)C(CCC1=CC=CC=C1)NC(C)C(=O)N2CCCC2C(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CCOC(=O)[C@H](CCC1=CC=CC=C1)N[C@@H](C)C(=O)N2CCC[C@H]2C(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C20H28N2O5 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
76095-16-4 (maleate) | |
| Record name | Enalapril [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0075847733 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID5022982 | |
| Record name | Enalapril | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5022982 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
376.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Enalapril | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014722 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
In water, 1.64X10+4 mg/L at 25 °C, 2.13e-01 g/L | |
| Record name | Enalapril | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00584 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Enalapril | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6529 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Enalapril | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014722 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
The renin-angiotensin-aldosterone system (RAAS) is a signaling pathway that works in synergism with the sympathetic system to regulate blood pressure and fluid and electrolyte homeostasis. Activation of this system upon stimulation by different factors, such as low blood pressure and nerve impulses, leads to increased release of norepinephrine (NE) from sympathetic nerve terminals and effects on the vascular growth, vasoconstriction, and salt retention in the kidneys. Renin is released from Renin acts on the precursor prottein angiotensinogen, which is a plasma globulin synthesized from the liver, to produce cleaved peptide hormone angiotensin I. Angiotensin I then can be further cleaved by ACE to produce angiotensin II, a vasoconstrictive peptide hormone. Present in different isoforms, angiotensin converting enzyme (ACE) is peptidyl dipeptidase enzyme expressed in various tissues, including the vascular tissues, such as the heart, brain, and kidneys. ACE also plays a role in inactivation of bradykinin, a potent vasodepressor peptide. Angiotensin II mediates various actions on the body by working on its G-protein coupled receptors, AT1 and AT2. It causes direct vasoconstriction of precapillary arterioles and postcapillary venules, inhibits the reuptake of NE thereby increasing available levels, stimulates the release of catecholamines from the adrenal medulla, reduces urinary excretion of sodium ions and water by promoting proximal tubular reabsorption, stimulates synthesis and release of aldosterone from the adrenal cortex, and stimulates hypertrophy of both vascular smooth muscle cells and cardiac myocytes. Enalapril is a pharmacologically inactive prodrug that requires hepatic biotransformation to form [enalaprilat], its active metabolite that works on the RAAS to inhibit ACE. Biotransformation is critial for the therapeutic actions of the drug, as enalapril itself is only a weak inhibitor of ACE. ACE inhibition results in reduced production and plasma levels of angiotensin II, increased plasma renin activity due to the loss of feedback inhibition by angiotensin II, and decreased aldosterone secretion. However, plasma aldosterone levels usually return to normal during long-term administration of enalapril. Decreased levels of angiotensin II subsequently leads to the dilatation of peripheral vessles and reduced vascular resistance which in turn lower blood pressure. While inhibition of ACE leading to suppression of RAAS is thought to be the primary mechanism of action of enalapril, the drug was shown to still exert antihypertensive effects on individuals with low-renin hypertension. It is suggested that enalapril may mediate its pharmacological actions via other modes of action that are not fully understood. As ACE is structurally similar to kininase I, which is a carboxypeptidase that degrades bradykinin, whether increased levels of bradykinin play a role in the therapeutic effects of enalapril remains to be elucidated., Enalapril maleate is a prodrug of enalaprilat and has little pharmacologic activity until hydrolyzed in vivo to enalaprilat. ... Enalapril prevents the conversion of angiotensin I to angiotensin II (a potent vasoconstrictor) through inhibition of angiotensin-converting enzyme (ACE). The drug competes with physiologic substrate (angiotensin I) for the active site of ACE; the affinity of enalaprilat for ACE is approximately 200,000 times greater than that of angiotensin I. In vitro on a molar basis, the affinity of enalaprilat for ACE is 300-1000 or 2-17 times that of enalapril or captopril, respectively. However, in vitro on a molar basis, the ACE-inhibitory effect of enalapril was shown to be similar to that of enalaprilat in rat plasma and kidneys, because these tissues extensively hydrolyze enalapril to form enalaprilat. The drug apparently does not inhibit brain ACE in animals. | |
| Record name | Enalapril | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00584 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Enalapril | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6529 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
CAS No. |
75847-73-3 | |
| Record name | Enalapril | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=75847-73-3 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Enalapril [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0075847733 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Enalapril | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00584 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Enalapril | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5022982 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | ENALAPRIL | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/69PN84IO1A | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Enalapril | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6529 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Enalapril | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014722 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
143-144.5, 143 - 144.5 °C | |
| Record name | Enalapril | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00584 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Enalapril | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014722 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods
Procedure details













Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: How does enalapril exert its antihypertensive effects?
A1: Enalapril, a prodrug, is metabolized to enalaprilat in the liver. Enalaprilat acts as a highly selective, competitive inhibitor of angiotensin-converting enzyme (ACE). [] This inhibition prevents the conversion of angiotensin I to angiotensin II, a potent vasoconstrictor. [, ] The reduction in angiotensin II levels leads to vasodilation, decreased aldosterone secretion, and ultimately, a decrease in blood pressure. [, , ]
Q2: Does enalapril affect tissue renin-angiotensin systems differently than other ACE inhibitors?
A2: Research suggests that enalapril may have differential effects on tissue ACE activities compared to other ACE inhibitors like captopril. [] In a study on rats with heart failure, enalapril, but not captopril, significantly decreased serum and lung ACE activities, while only captopril inhibited aortic ACE activity. [] This suggests potential for targeted effects depending on the ACE inhibitor used.
Q3: How does enalapril impact cardiac sympathetic activity in heart failure patients?
A3: Studies using [123I]-metaiodobenzylguanidine (MIBG), a norepinephrine analog, suggest that enalapril improves cardiac sympathetic neuronal uptake function in heart failure patients. [] This improvement was observed alongside a lack of significant decrease in plasma norepinephrine concentrations, suggesting a targeted effect on cardiac neurons. []
Q4: What is the molecular formula and weight of enalapril?
A4: The molecular formula of enalapril maleate is C20H28N2O5 · C4H4O4, and its molecular weight is 492.5 g/mol.
Q5: Are there any specific material compatibility or stability issues associated with enalapril?
A5: While the provided research papers primarily focus on the pharmacological aspects of enalapril, information regarding material compatibility and stability under various conditions is limited. Further investigation into these aspects might be necessary for specific applications beyond its pharmaceutical use.
Q6: How is enalapril absorbed and metabolized in the body?
A7: Enalapril is administered orally and is rapidly absorbed from the gastrointestinal tract. [] It undergoes hydrolysis in the liver to its active metabolite, enalaprilat, which is a more potent ACE inhibitor than enalapril itself. [, , ]
Q7: Does probenecid affect enalapril pharmacokinetics?
A8: Yes, probenecid pretreatment has been shown to increase both the peak serum concentration and area under the curve (AUC) of enalapril and enalaprilat, while decreasing their renal clearance and total drug recovery. [] This suggests that probenecid may interfere with the renal excretion of enalapril and its active metabolite.
Q8: Does enalapril exhibit a linear pharmacokinetic profile?
A9: While enalapril generally exhibits linear pharmacokinetics, research suggests that factors like serum creatinine, severity of heart failure, basal metabolic rate, and body weight can influence enalaprilat trough levels, particularly in chronic heart failure patients. [] This highlights the importance of individual dose adjustments in certain patient populations.
Q9: What is the efficacy of enalapril compared to other antihypertensive agents like nifedipine and losartan?
A9: Multiple studies have compared the efficacy of enalapril to other antihypertensive medications.
- Enalapril vs. Metoprolol: A study comparing enalapril and metoprolol in patients with mild to moderate essential hypertension found both drugs to be safe and effective as initial therapy. [] Both treatments significantly reduced blood pressure, with enalapril consistently showing a greater reduction in systolic blood pressure. []
- Enalapril vs. Ramipril: In a study comparing enalapril and ramipril in patients with mild-to-moderate essential hypertension, ramipril exhibited a greater reduction in 24-hour ambulatory diastolic and systolic blood pressure. []
Q10: Are there any known resistance mechanisms to enalapril?
A10: While the research provided does not explicitly mention specific resistance mechanisms to enalapril, it's important to note that individual patient response to ACE inhibitors can vary. Factors influencing efficacy may include genetic polymorphisms affecting the renin-angiotensin system, concomitant medications, and underlying comorbidities.
Q11: What analytical techniques are used to determine enalapril and enalaprilat concentrations?
A11: Various analytical methods have been developed and validated for the quantitative determination of enalapril and enalaprilat in biological samples, including:
- Liquid chromatography–tandem mass spectrometry (LC-MS/MS): This highly sensitive and specific method has been widely employed to quantify enalapril and enalaprilat in human plasma. [, , ]
- High-performance liquid chromatography (HPLC) coupled with various detection methods: HPLC with ultraviolet (UV) detection and HPLC-MS have been used to analyze enalapril in different matrices, including Caco-2 cell monolayers. [, ]
Q12: What are the typical validation parameters for these analytical methods?
A15: Analytical methods for enalapril and enalaprilat are rigorously validated for accuracy, precision, linearity, sensitivity, selectivity, recovery, and stability. [, , ] These parameters ensure the reliability and reproducibility of the analytical data generated.
Q13: Has enalapril been investigated for potential applications beyond hypertension and heart failure?
A16: While the provided research focuses primarily on cardiovascular applications of enalapril, its influence on various physiological processes like inflammation and angiogenesis has prompted investigations in other areas, including diabetic retinopathy. [] Preclinical studies have shown enalapril to potentially mitigate retinal damage by altering the expression of key growth factors involved in diabetic retinopathy, highlighting its potential therapeutic breadth. []
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.