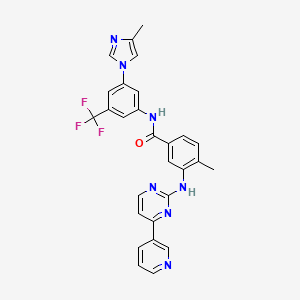
Nilotinib
Vue d'ensemble
Description
Le nilotinib est un inhibiteur de tyrosine kinase de deuxième génération principalement utilisé pour le traitement de la leucémie myéloïde chronique (LMC) positive pour le chromosome de Philadelphie. Il est commercialisé sous le nom de marque Tasigna. Le this compound agit en inhibant la tyrosine kinase BCR-ABL, qui est produite par l'anomalie chromosomique de Philadelphie et est responsable de la prolifération incontrôlée des cellules leucémiques .
Mécanisme D'action
Target of Action
Nilotinib is a transduction inhibitor that primarily targets BCR-ABL , c-kit , and PDGF . These targets play a crucial role in the development of various leukemias, including chronic myeloid leukemia (CML) . The BCR-ABL oncogene, in particular, is responsible for causing chronic myelogenous leukemia .
Mode of Action
This compound works by inhibiting the tyrosine kinase activity of the BCR-ABL protein . It binds to the ATP-binding site of the inactive conformation of ABL with a greater affinity than imatinib . This binding stabilizes the inactive conformation, thereby inhibiting the abnormal protein that signals cancer cells to multiply . This action helps to stop or slow the spread of cancer cells .
Biochemical Pathways
The primary biochemical pathway affected by this compound is the BCR-ABL pathway . This compound inhibits the tyrosine kinase activity of the BCR-ABL protein, which is a product of the BCR-ABL oncogene . This inhibition disrupts the signaling within leukemia cancer cells, thereby slowing or stopping their multiplication .
Pharmacokinetics
This compound is rapidly absorbed, with a peak serum concentration approximately 3 hours after dosing . The metabolism of this compound is primarily via hepatic cytochrome P450 (CYP) 3A4 . Exposure to this compound can be significantly reduced by the induction of CYP3A4 with rifampicin and significantly increased by the inhibition of CYP3A with ketoconazole . The bioavailability of this compound is increased by up to 82% when given with a high-fat meal compared with a fasted state .
Result of Action
The molecular and cellular effects of this compound’s action include the alteration of LPS-induced proinflammatory/anti-inflammatory cytokine mRNA levels by suppressing AKT/P38/SOD2 signaling . This compound treatment also significantly downregulates LPS-stimulated proinflammatory cytokine levels in primary microglia and primary astrocytes by altering P38/STAT3 signaling . In addition, this compound significantly reduces proinflammatory cytokine IL-1β, IL-6, and COX-2 levels and P38/STAT3 signaling in the brain in LPS-treated wild-type mice .
Action Environment
Environmental factors can influence the action, efficacy, and stability of this compound. For instance, the bioavailability of this compound is increased by up to 82% when given with a high-fat meal compared with a fasted state . This suggests that dietary factors can significantly influence the pharmacokinetics and, consequently, the efficacy of this compound. Moreover, the exposure to this compound can be significantly reduced by the induction of CYP3A4 with rifampicin and significantly increased by the inhibition of CYP3A with ketoconazole , indicating that the presence of other medications can also impact the action of this compound.
Applications De Recherche Scientifique
Nilotinib has a wide range of scientific research applications, particularly in the fields of chemistry, biology, medicine, and industry. In medicine, this compound is used to treat chronic myeloid leukemia by inhibiting the BCR-ABL tyrosine kinase activity and preventing the proliferation of leukemic cells . Additionally, this compound analogues have been synthesized and evaluated for their antiplatelet activity and functionality towards cancer cell proliferation . In chemistry, this compound is used as a model compound for studying the synthesis and characterization of tyrosine kinase inhibitors. In industry, this compound is used in the development of new pharmaceutical formulations and drug delivery systems .
Méthodes De Préparation
Le nilotinib peut être synthétisé par différentes méthodes. Une voie de synthèse courante implique la conversion d'un composé de formule (IV) en chlorhydrate de this compound. Ce processus implique l'utilisation de réactifs et de conditions spécifiques pour assurer la stabilité et la pureté du produit final . Une autre méthode implique la préparation de compositions pharmaceutiques granulaires sèches de this compound par compactage du chlorhydrate de this compound avec des excipients pharmaceutiquement acceptables . De plus, une méthode de nano-préparation implique l'utilisation de N, N-diméthylformamide et de polyvinylpyrrolidone pour créer une suspension de nano-médicament stable, qui est ensuite séchée par pulvérisation pour obtenir le produit final .
Analyse Des Réactions Chimiques
Le nilotinib subit diverses réactions chimiques, notamment l'oxydation, la réduction et la substitution. Les réactifs couramment utilisés dans ces réactions comprennent les oxydants, les réducteurs et les nucléophiles. Par exemple, le this compound peut être synthétisé par formation de benzanilide dans l'eau en utilisant la chimie de ligation chimique native (NCL) . Cette méthode implique le couplage de fragments de benzoyle et de mercaptoaniline pour former des liaisons amides aromatiques. Les principaux produits formés à partir de ces réactions comprennent divers dérivés du this compound avec des applications thérapeutiques potentielles.
Applications de recherche scientifique
Le this compound a un large éventail d'applications de recherche scientifique, en particulier dans les domaines de la chimie, de la biologie, de la médecine et de l'industrie. En médecine, le this compound est utilisé pour traiter la leucémie myéloïde chronique en inhibant l'activité de la tyrosine kinase BCR-ABL et en empêchant la prolifération des cellules leucémiques . De plus, des analogues du this compound ont été synthétisés et évalués pour leur activité antiplaquettaire et leur fonctionnalité vis-à-vis de la prolifération des cellules cancéreuses . En chimie, le this compound est utilisé comme composé modèle pour étudier la synthèse et la caractérisation des inhibiteurs de tyrosine kinase. Dans l'industrie, le this compound est utilisé dans le développement de nouvelles formulations pharmaceutiques et de systèmes d'administration de médicaments .
Mécanisme d'action
Le this compound exerce ses effets en inhibant sélectivement la tyrosine kinase BCR-ABL, c-KIT et le récepteur du facteur de croissance dérivé des plaquettes (PDGFR) . Il se lie au site de liaison de l'ATP de BCR-ABL et inhibe son activité de tyrosine kinase, empêchant ainsi la phosphorylation et l'activation des voies de signalisation en aval impliquées dans la prolifération et la survie cellulaires. Le this compound est particulièrement efficace dans les cas de leucémie myéloïde chronique résistante à l'imatinib, un autre inhibiteur de tyrosine kinase .
Comparaison Avec Des Composés Similaires
Le nilotinib est souvent comparé à d'autres inhibiteurs de tyrosine kinase tels que l'imatinib et le dasatinib. Alors que l'imatinib a été le premier inhibiteur de tyrosine kinase approuvé pour le traitement de la leucémie myéloïde chronique, le this compound et le dasatinib sont des inhibiteurs de deuxième génération avec des profils d'efficacité et de sécurité améliorés . Le this compound a une affinité de liaison plus élevée pour la tyrosine kinase BCR-ABL et est efficace contre un éventail plus large de mutations BCR-ABL par rapport à l'imatinib . Le dasatinib, quant à lui, est structurellement distinct du this compound et a un spectre d'activité différent contre les mutants du domaine kinase BCR-ABL . D'autres composés similaires comprennent le ponatinib, qui est efficace contre la mutation T315I résistante à la fois au this compound et au dasatinib .
Propriétés
IUPAC Name |
4-methyl-N-[3-(4-methylimidazol-1-yl)-5-(trifluoromethyl)phenyl]-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]benzamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C28H22F3N7O/c1-17-5-6-19(10-25(17)37-27-33-9-7-24(36-27)20-4-3-8-32-14-20)26(39)35-22-11-21(28(29,30)31)12-23(13-22)38-15-18(2)34-16-38/h3-16H,1-2H3,(H,35,39)(H,33,36,37) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
HHZIURLSWUIHRB-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1=C(C=C(C=C1)C(=O)NC2=CC(=CC(=C2)C(F)(F)F)N3C=C(N=C3)C)NC4=NC=CC(=N4)C5=CN=CC=C5 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C28H22F3N7O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID5042663 | |
| Record name | Nilotinib | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5042663 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
529.5 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Nilotinib | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015595 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
The solubility ... in aqueous solutions decreases with increasing pH, 2.01e-03 g/L | |
| Record name | Nilotinib | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7842 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Nilotinib | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015595 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Chronic myelogenous leukaemia (CML) is caused by the BCR-ABL oncogene. Nilotinib inhibits the tyrosine kinase activity of the BCR-ABL protein. Nilotinib fits into the ATP-binding site of the BCR-ABL protein with higher affinity than imatinib, over-riding resistance caused by mutations. The ability of AMN107 to inhibit TEL-platelet-derived growth factor receptor-beta (TEL-PDGFRbeta), which causes chronic myelomonocytic leukaemia, and FIP1-like-1-PDGFRalpha, which causes hypereosinophilic syndrome, suggests potential use of AMN107 for myeloproliferative diseases characterised by these kinase fusions (Stover et al, 2005; Weisberg et al, 2005). AMN107 also inhibits the c-Kit receptor kinase, including the D816V-mutated variant of KIT, at pharmacologically achievable concentrations, supporting potential utility in the treatment of mastocytosis, and gastrointestinal stromal tumours (Weisberg et al, 2005; von Bubnoff et al, 2005; Gleixner et al, 2006)., Nilotinib, an inhibitor of Bcr-Abl tyrosine kinase, is an antineoplastic agent. Chronic myelogenous leukemia (CML) is a clonal myeloproliferative disorder characterized by the expansion of hematopoietic cells carrying the Philadelphia chromosome (Ph), resulting from a reciprocal translocation of the long arms of chromosomes 9 and 22. A novel fusion gene is formed, Bcr-Abl, which encodes a constitutively active, cytoplasmic form of protein tyrosine kinase. The unregulated activity of the Abl tyrosine kinase in Bcr-Abl is the cause of CML. Nilotinib is an orally active aminopyrimidine-derivative tyrosine kinase inhibitor that functions through competitive inhibition at the ATP-binding site of Bcr-Abl, leading to the inhibition of tyrosine phosphorylation of proteins that are involved in the intracellular signal transduction that Bcr-Abl mediates., Clinical resistance to imatinib in CML has been attributed to several mechanisms, but point mutations in the Bcr-Abl kinase domain appear to the most common, occurring in 30-90% of patients who develop resistance. The ability of nilotinib to overcome imatinib resistance resulting from Bcr-Abl kinase domain mutations has been demonstrated in vitro. In preclinical studies in cell-line models, nilotinib inhibited most (32 of 33) imatinib-resistant Bcr-Abl kinase domain mutant forms., Nilotinib binds to and stabilizes the inactive conformation of the kinase domain of Abl protein. In vitro, nilotinib inhibited Bcr-Abl mediated proliferation of murine leukemic cell lines and human cell lines derived from patients with Ph+ CML. Under the conditions of the assays, nilotinib was able to overcome imatinib resistance resulting from Bcr-Abl kinase mutations, in 32 out of 33 mutations tested. In vivo, nilotinib reduced the tumor size in a murine Bcr-Abl xenograft model. Nilotinib inhibited the autophosphorylation of the following kinases at IC50 values as indicated: Bcr-Abl (20-60 nM), PDGFR (69 nM), c-Kit (210 nM), CSF-1R (125-250 nM) and DDR (3.7 nM)., It is an important challenge to better understand the mechanisms of tyrosine kinase inhibitors-induced apoptosis in CML cells. Thus, /the authors/ have investigated how this apoptosis can be modulated by extracellular factors. Apoptosis induced by imatinib and nilotinib was determined in BCR-ABL expressing cell lines and primary CML CD34+ cells. Both molecules induced apoptosis of BCR-ABL expressing cells. This apoptosis was inhibited by protein synthesis inhibition in both K562 and CML CD34+ cells. In K562, 80% inhibition of the BCR-ABL auto-phosphorylation by either imatinib or nilotinib induced a two fold increase in Bim-EL expression and induction of apoptosis in 48 hr. Bim accumulation preceded apoptosis induction which was completely abolished by depletion in Bim using shRNA. However, the anti-proliferative effect of imatinib was preserved in Bim-depleted cells. When K562 cells were cultured in a cytokine containing medium, the pro-apoptotic effect of nilotinib was decreased by 68% and this was related to a decrease in Bim-EL dephosphorylation and accumulation. Similarly, the presence of a combination of cytokines inhibited 88% of NIL- and 39% of IMA-induced apoptosis in primary CML CD34+ cells. In conclusion, both nilotinib and imatinib induce apoptosis through Bim accumulation independently of cell cycle arrest. However, the pro-apoptotic effect of both molecules can be attenuated by the presence of cytokines and growth factors, particularly concerning nilotinib. Thus BCR-ABL inhibition restores the cytokine dependence but is not sufficient to induce apoptosis when other signaling pathways are activated., For more Mechanism of Action (Complete) data for Nilotinib (7 total), please visit the HSDB record page. | |
| Record name | Nilotinib | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB04868 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Nilotinib | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7842 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
White to slightly yellowish to slightly greenish yellow powder | |
CAS No. |
641571-10-0, 923288-90-8 | |
| Record name | Nilotinib | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=641571-10-0 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Nilotinib [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0641571100 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Nilotinib | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB04868 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Nilotinib | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=747599 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Nilotinib | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5042663 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)phenyl]-3-{[4-(pyridin-3-yl)pyrimidin-2-yl]amino}benzamide | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.166.395 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | 4-Methyl-N-(3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)-phenyl)-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)benzamide | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | NILOTINIB | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/F41401512X | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Nilotinib | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7842 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Nilotinib | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015595 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods I
Procedure details








Synthesis routes and methods II
Procedure details







Synthesis routes and methods III
Procedure details





Synthesis routes and methods IV
Procedure details











Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: How does Nilotinib interact with its target, and what are the downstream effects of this interaction?
A1: this compound functions as a potent and selective inhibitor of the Bcr-Abl tyrosine kinase, a constitutively active kinase arising from the Philadelphia chromosome translocation found in chronic myeloid leukemia (CML). [, , ] It exerts its effect by competitively binding to the ATP-binding site of Bcr-Abl, thereby inhibiting tyrosine phosphorylation of downstream signaling proteins. [, ] This blockage disrupts critical pathways like JAK-STAT, cell cycle progression, and ABC transporter activity, ultimately leading to the inhibition of proliferation and induction of apoptosis in Bcr-Abl-expressing cells. [, , ]
Q2: What is the molecular formula and weight of this compound?
A2: The molecular formula of this compound is C28H22F3N7O, and its molecular weight is 539.5 g/mol. []
Q3: Is there any spectroscopic data available for this compound?
A3: While the provided research papers don't delve into detailed spectroscopic characterization, high-performance liquid chromatography is mentioned as a technique for measuring this compound plasma trough concentrations. []
Q4: What evidence supports the efficacy of this compound in treating CML?
A4: Numerous studies highlight this compound's efficacy in treating CML. Clinical trials like ENESTnd demonstrate its superiority over Imatinib in achieving higher rates of cytogenetic and molecular responses in newly diagnosed CML patients. [, , ] Further evidence stems from Phase II trials showcasing its effectiveness in Imatinib-resistant or -intolerant CML patients, achieving significant hematologic and cytogenetic responses. [, , ]
Q5: Are there any in vitro studies that support this compound's efficacy?
A5: Yes, in vitro studies using CML cell lines like K562 reveal this compound's potent inhibitory effects. It effectively inhibits Bcr-Abl autophosphorylation and proliferation at significantly lower concentrations compared to Imatinib, indicating its higher potency. [] Moreover, it effectively suppresses CML progenitor cell growth, primarily by inhibiting cell division. []
Q6: What are the known mechanisms of resistance to this compound in CML?
A6: Despite its efficacy, resistance to this compound can arise through various mechanisms. One major contributor is the emergence of mutations within the Bcr-Abl kinase domain, such as the T315I mutation, which hinders this compound binding. [, , , ] Other mechanisms include overexpression of anti-apoptotic proteins like BCL2 and upregulation of drug efflux pumps like MRP1, contributing to decreased intracellular drug concentrations. []
Q7: Does cross-resistance exist between this compound and other TKIs like Imatinib or Dasatinib?
A7: Yes, cross-resistance can occur, particularly with mutations in the Bcr-Abl kinase domain. While this compound demonstrates efficacy against many Imatinib-resistant mutations, certain mutations, like T315I, confer resistance to both. [, , , ] Interestingly, Dasatinib, due to its distinct binding mechanism, retains efficacy against some this compound-resistant mutations, including those affecting the F359 residue. []
Q8: Does this compound interact with drug transporters?
A9: Yes, research indicates that this compound interacts with the ABCB1 (MDR1) transporter, a drug efflux pump. [] This interaction can potentially impact its tissue distribution and efficacy, as ABCB1 actively transports substrates out of cells. []
Q9: What is the safety profile of this compound, and are there any potential long-term effects?
A10: While generally well-tolerated, this compound can cause adverse effects. Common side effects include rash, nausea, pruritus, and hematological abnormalities like neutropenia and thrombocytopenia. [, , ] Additionally, it has been linked to metabolic changes, including hyperglycemia, hyperlipidemia, and an increased risk of arterial occlusive events like peripheral artery disease. [, , ] Long-term use necessitates careful monitoring for these potential complications.
Q10: Are there any strategies to improve this compound delivery to specific targets or tissues?
A10: While the provided research papers primarily focus on this compound's systemic administration, future research could explore targeted delivery strategies. Nanocarrier-based drug delivery systems, for instance, hold potential for enhancing its accumulation in specific tissues like the bone marrow, potentially improving efficacy and minimizing off-target effects.
Q11: What biomarkers are being investigated for predicting this compound efficacy or monitoring treatment response?
A12: Monitoring BCR-ABL transcript levels via RT-PCR serves as a crucial biomarker for assessing treatment response and identifying potential resistance. [, , , ] Achieving deep molecular responses, like MR4.5 (BCR-ABL ≤ 0.0032%IS), is associated with favorable outcomes. [, ] Additionally, plasma trough concentrations of this compound have been correlated with molecular response, suggesting a potential role in treatment optimization. []
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.
![N-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)nicotinamide (7,7-dimethyl-2-oxobicyclo[2.2.1]heptan-1-yl)methanesulfonate](/img/structure/B1678813.png)