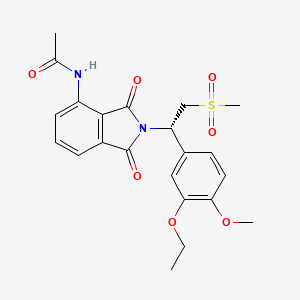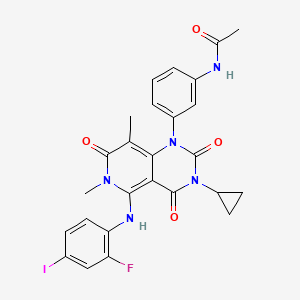
Trametinib
Vue d'ensemble
Description
Trametinib est un inhibiteur de la kinase activée par les mitogènes (MAPK) kinase (MEK) biodisponible par voie orale ayant une activité antinéoplasique. Il se lie spécifiquement à MEK1 et MEK2, ce qui entraîne l'inhibition de la signalisation cellulaire induite par les facteurs de croissance et la prolifération cellulaire dans divers cancers . This compound est principalement utilisé pour le traitement du mélanome non résécable ou métastatique présentant des mutations BRAF V600E ou V600K .
Applications De Recherche Scientifique
Trametinib has a wide range of scientific research applications:
Chemistry: It is used as a model compound in the study of kinase inhibitors and their mechanisms of action.
Biology: This compound is used to study cell signaling pathways, particularly the MAPK/ERK pathway.
Industry: This compound is used in the pharmaceutical industry for the development of new cancer therapies.
Mécanisme D'action
Target of Action
Trametinib is a kinase inhibitor that primarily targets mitogen-activated extracellular signal-regulated kinase 1 (MEK1) and MEK2 . These kinases play a crucial role in the MAPK/ERK pathway , which is involved in cell proliferation, survival, differentiation, and angiogenesis .
Mode of Action
This compound functions as an allosteric, ATP non-competitive inhibitor . It binds to unphosphorylated MEK1 and MEK2 with high affinity, blocking their catalytic activity . This inhibition prevents the phosphorylation and activation of MEKs, thereby suppressing downstream ERK signaling .
Biochemical Pathways
The primary biochemical pathway affected by this compound is the RAS/RAF/MEK/ERK pathway . This pathway transduces signals from various extracellular stimuli, leading to distinct intracellular responses . This compound’s inhibition of MEK1 and MEK2 disrupts this pathway, reducing the proliferation and survival of tumor cells .
Pharmacokinetics
This compound is orally bioavailable . Following oral administration, over 80% of excreted radioactivity was recovered in the feces, while less than 20% was recovered in the urine, with less than 0.1% of the excreted dose as the parent molecule . This suggests that this compound is primarily eliminated via fecal excretion.
Result of Action
This compound’s action results in a decrease in cell proliferation and an increase in apoptosis . It has been shown to induce G1 cell cycle arrest in vitro . Additionally, this compound significantly decreases macrophage infiltration and the expression of pro-inflammatory cytokines in the kidneys . It also lowers lipid peroxidation by-products, restores the reduced glutathione/oxidized glutathione ratio, and downregulates NADPH oxidase 4 .
Action Environment
The efficacy of this compound can be influenced by environmental factors such as patient adherence to the medication regimen . Poor adherence can result in suboptimal drug exposure and consequently unfavorable patient outcomes . Therefore, ensuring patient adherence to this compound is crucial for its therapeutic success.
Analyse Biochimique
Biochemical Properties
Trametinib specifically binds to MEK1 and MEK2, resulting in inhibition of growth factor-mediated cell signaling and cellular proliferation in various cancers . It is a reversible, highly selective, allosteric inhibitor of MEK1 and MEK2 . By binding to unphosphorylated MEK1 and MEK2 with high affinity, this compound blocks the catalytic activity of MEKs .
Cellular Effects
This compound has been shown to inhibit the proliferation, migration, and invasion of glioma cells, while inducing apoptosis of glioma cells . It can suppress both the expression of PKM2 in glioma cells and the transport of PKM2 into the cellular nucleus via suppression of ERK1/2 expression . This compound also significantly reduces the phosphorylation of MEK1/2 and extracellular signal-regulated kinase 1/2 (ERK1/2), mitigated renal dysfunction, and ameliorated histopathological abnormalities .
Molecular Mechanism
This compound is a kinase inhibitor that inhibits cell growth of various BRAF V600 mutation-positive tumors in vitro and in vivo . It functions as an allosteric, ATP noncompetitive inhibitor with nanomolar activity against both MEK 1 and MEK 2 kinases . It maintains MEK in an unphosphorylated form, preventing phosphorylation and activation of MEKs .
Temporal Effects in Laboratory Settings
This compound has been shown to have significant effects over time in laboratory settings. For instance, it has been found that this compound can inhibit the growth and intracellular glycolysis of glioma cells by targeting the PKM2/c-Myc pathway . Moreover, this compound treatment accelerated disease onset and decreased epidermal thickness, which was in large part ameliorated by Losartan treatment .
Dosage Effects in Animal Models
The effects of this compound vary with different dosages in animal models. For instance, this compound has been shown to significantly enhance remyelination in both MOG-induced EAE model and LPC-induced focal demyelination model . Furthermore, this compound has been shown to inhibit the growth of the transplanted glioma cell tumor .
Metabolic Pathways
This compound is involved in the MAPK pathway, which plays a critical role in cell growth, differentiation, inflammation, and apoptosis . Mutant BRAF proteins signal through MEK1 and MEK2, stimulating cell growth . This compound is metabolized predominantly via deacetylation followed by oxidation and/or glucuronidation .
Transport and Distribution
This compound has limited brain distribution due to active efflux at the blood-brain barrier (BBB) . Following administration, this compound and its metabolites are excreted in the feces (≥81%) and to a minor extent in urine (≤19%) .
Subcellular Localization
This compound is mainly localized in the cytoplasm . It has been shown that this compound can suppress both the expression of PKM2 in glioma cells and the transport of PKM2 into the cellular nucleus via suppression of ERK1/2 expression .
Méthodes De Préparation
Voies de synthèse et conditions de réaction
La synthèse de trametinib implique plusieurs étapes clés. Le matériau de départ est généralement une aniline substituée, qui subit une série de réactions, notamment la cyclisation, l'acylation et l'halogénation, pour former le produit final . Les conditions de réaction impliquent souvent l'utilisation de solvants organiques tels que le diméthylsulfoxyde et de réactifs tels que le chlorure de thionyle .
Méthodes de production industrielle
En milieu industriel, la production de this compound est mise à l'échelle en utilisant des voies de synthèse similaires, mais avec des conditions de réaction optimisées afin de garantir un rendement et une pureté élevés. Le processus implique des mesures rigoureuses de contrôle de la qualité pour surveiller la formation d'impuretés et s'assurer que le produit final répond aux normes réglementaires .
Analyse Des Réactions Chimiques
Types de réactions
Trametinib subit diverses réactions chimiques, notamment :
Oxydation : This compound peut être oxydé dans certaines conditions, ce qui conduit à la formation de produits de dégradation.
Réduction : Des réactions de réduction peuvent également se produire, bien qu'elles soient moins fréquentes.
Substitution : Les réactions de substitution, en particulier celles impliquant des atomes d'halogène, sont une partie essentielle du processus de synthèse.
Réactifs et conditions courants
Oxydation : Les oxydants courants comprennent le peroxyde d'hydrogène et le permanganate de potassium.
Réduction : Des agents réducteurs tels que le borohydrure de sodium peuvent être utilisés.
Substitution : Des réactifs d'halogénation comme le chlorure de thionyle sont couramment utilisés.
Principaux produits formés
Les principaux produits formés à partir de ces réactions comprennent divers intermédiaires et impuretés, tels que le this compound désacétylé et l'impureté de cyclopropanamide .
Applications de la recherche scientifique
This compound a un large éventail d'applications dans la recherche scientifique :
Mécanisme d'action
This compound exerce ses effets en inhibant l'activité de MEK1 et MEK2, qui sont des composants clés de la voie de signalisation MAPK/ERK . Cette voie est impliquée dans la régulation de la prolifération, de la survie, de la différenciation et de la motilité cellulaires . En inhibant MEK1 et MEK2, this compound empêche la phosphorylation et l'activation des molécules de signalisation en aval, ce qui conduit à l'inhibition de la croissance des cellules tumorales .
Comparaison Avec Des Composés Similaires
Composés similaires
Cobimétinib : Un autre inhibiteur de MEK utilisé en association avec le vémurafenib pour le traitement du mélanome.
Selumétinib : Un inhibiteur de MEK utilisé pour le traitement de la neurofibromatose de type 1.
Unicité du trametinib
This compound est unique en son genre par sa capacité à inhiber sélectivement à la fois MEK1 et MEK2 avec une grande puissance . Il a montré une efficacité significative en association avec les inhibiteurs de BRAF, tels que le dabrafenib, pour le traitement des cancers BRAF-mutants . Cette thérapie combinée s'est avérée fournir une inhibition plus importante et plus prolongée de la croissance tumorale par rapport à l'un ou l'autre médicament administré seul .
Propriétés
IUPAC Name |
N-[3-[3-cyclopropyl-5-(2-fluoro-4-iodoanilino)-6,8-dimethyl-2,4,7-trioxopyrido[4,3-d]pyrimidin-1-yl]phenyl]acetamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C26H23FIN5O4/c1-13-22-21(23(31(3)24(13)35)30-20-10-7-15(28)11-19(20)27)25(36)33(17-8-9-17)26(37)32(22)18-6-4-5-16(12-18)29-14(2)34/h4-7,10-12,17,30H,8-9H2,1-3H3,(H,29,34) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
LIRYPHYGHXZJBZ-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1=C2C(=C(N(C1=O)C)NC3=C(C=C(C=C3)I)F)C(=O)N(C(=O)N2C4=CC=CC(=C4)NC(=O)C)C5CC5 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C26H23FIN5O4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID901007381 | |
| Record name | N-{3-[3-Cyclopropyl-5-(2-fluoro-4-iodoanilino)-6,8-dimethyl-2,4,7-trioxo-3,4,6,7-tetrahydropyrido[4,3-d]pyrimidin-1(2H)-yl]phenyl}ethanimidic acid | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID901007381 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
615.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Mechanism of Action |
Trametinib is a reversible, allosteric inhibitor of mitogen-activated extracellular signal regulated kinase 1 _(MEK1)_ and _MEK2_ activation and of_ MEK1_ and _MEK2_ kinase activity. MEK proteins are upstream regulators of the extracellular signal-related kinase (ERK) pathway, which promotes cellular proliferation. Trametinib helps with melanoma with the BRAF V600E or V600K as the mutation results in the constitutive activation of the BRAF pathway which includes MEK1 and MEK2. | |
| Record name | Trametinib | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08911 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
871700-17-3 | |
| Record name | Trametinib | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=871700-17-3 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Trametinib [USAN:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0871700173 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Trametinib | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08911 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | N-{3-[3-Cyclopropyl-5-(2-fluoro-4-iodoanilino)-6,8-dimethyl-2,4,7-trioxo-3,4,6,7-tetrahydropyrido[4,3-d]pyrimidin-1(2H)-yl]phenyl}ethanimidic acid | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID901007381 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | N-{3-[3-cyclopropyl-5-[(2-fluoro-4-iodophenyl)amino]-6,8-dimethyl-2,4,7-trioxo-3,4,6,7-tetrahydropyrido[4,3-d]pyrimidin-1(2H)-yl]phenyl}acetamide | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | TRAMETINIB | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/33E86K87QN | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Melting Point |
293-303 | |
| Record name | Trametinib | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08911 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Synthesis routes and methods I
Procedure details








Synthesis routes and methods II
Procedure details








Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.