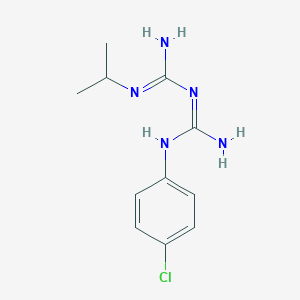
Proguanil
Vue d'ensemble
Description
Proguanil D6: est un dérivé deutéré du this compound, un agent antipaludique. Le this compound est largement utilisé pour la prophylaxie et le traitement du paludisme causé par Plasmodium falciparum et Plasmodium vivax . Le marquage au deutérium dans le this compound D6 améliore sa stabilité et permet des études pharmacocinétiques plus précises .
Applications De Recherche Scientifique
Proguanil D6 has a wide range of scientific research applications, including:
Biology: Employed in biological studies to understand the mechanism of action of antimalarial drugs.
Medicine: Investigated for its potential anticancer properties, particularly in breast and bladder cancer
Industry: Utilized in the development of new antimalarial formulations and drug delivery systems.
Mécanisme D'action
Target of Action
Proguanil primarily targets the dihydrofolate reductase enzyme in the malaria parasites, Plasmodium falciparum and Plasmodium vivax . This enzyme plays a crucial role in the reproduction of the parasite .
Mode of Action
This compound inhibits the dihydrofolate reductase of plasmodia, thereby blocking the biosynthesis of purines and pyrimidines, which are essential for DNA synthesis and cell multiplication . This leads to the failure of nuclear division at the time of schizont formation in erythrocytes and liver .
Biochemical Pathways
The inhibition or disruption of folate metabolism is an attractive target for the discovery of new antimalarial drugs . This compound is converted in vivo to the active metabolite, cycloguanil, an inhibitor of the dihydrofolate reductase enzyme . This pathway is critical to the parasite’s survival .
Pharmacokinetics
This compound is well absorbed after oral dosage . The elimination half-life of this compound and its principal metabolite, cycloguanil, is 12 to 15 hours in adults and children . About 60% of a dose of this compound is excreted unchanged in the urine . This compound is essentially a pro-drug as it is metabolized to cycloguanil and 4-chlorophenyl-biguanide, the former being a potent antimalarial compound .
Result of Action
The result of this compound’s action is the prevention and suppression of malaria caused by susceptible strains of P. falciparum and other species of Plasmodium found in some geographical areas of the world . It has causal prophylactic and suppressive activity against P. falciparum and cures the acute infection .
Action Environment
The action, efficacy, and stability of this compound can be influenced by various environmental factors. For instance, dietary fat increases the rate and extent of atovaquone absorption, a drug often used in combination with this compound . Furthermore, this compound is rapidly and extensively absorbed regardless of food intake . .
Analyse Biochimique
Biochemical Properties
Proguanil is a biguanide derivative that is converted to an active metabolite called cycloguanil . It exerts its antimalarial action by inhibiting the enzyme, dihydrofolate reductase, which is involved in the reproduction of the malaria parasite, Plasmodium falciparum and Plasmodium vivax . This inhibition blocks the biosynthesis of purines and pyrimidines, which are essential for DNA synthesis and cell multiplication .
Cellular Effects
This compound works by stopping the malaria parasite, Plasmodium falciparum and Plasmodium vivax, from reproducing once it is in the red blood cells . It does this by inhibiting the enzyme, dihydrofolate reductase, which is involved in the reproduction of the parasite . This leads to failure of nuclear division at the time of schizont formation in erythrocytes and liver .
Molecular Mechanism
The molecular mechanism of this compound involves the inhibition of the enzyme dihydrofolate reductase of plasmodia . This inhibition blocks the biosynthesis of purines and pyrimidines, which are essential for DNA synthesis and cell multiplication . This leads to failure of nuclear division at the time of schizont formation in erythrocytes and liver .
Temporal Effects in Laboratory Settings
This compound has been shown to have potent, but slow-acting, in vitro anti-plasmodial activity . The potent fast-acting activity of this compound is attributed to the dihydrofolate reductase inhibitor cycloguanil .
Dosage Effects in Animal Models
While specific dosage effects of this compound in animal models were not found in the search results, it is known that this compound is extensively absorbed in rats . In both species, toxicity was related to this compound exposure, the principal manifestations being salivation, emesis, and loss of body weight .
Metabolic Pathways
This compound is variably metabolized in the liver by cytochrome P450 isoenzymes to the active triazine metabolite, cycloguanil . This variable metabolism of this compound may have profound clinical importance in poor metabolizers such as the Asian and African populations at risk for malaria infection .
Transport and Distribution
This compound and its metabolite cycloguanil were found to be substrates of organic cation transporter 1 (OCT1), organic cation transporter 2 (OCT2), multidrug and toxin extrusion 1 (MATE1) and multidrug and toxin extrusion 2-K (MATE2-K) . These transporters play a crucial role in the distribution and excretion of this compound .
Subcellular Localization
The specific subcellular localization of this compound was not found in the search results. Given its mechanism of action, it can be inferred that this compound likely localizes to the site of the enzyme dihydrofolate reductase, which is involved in the reproduction of the malaria parasite .
Méthodes De Préparation
Voies de Synthèse et Conditions de Réaction: : La synthèse du Proguanil D6 implique l'incorporation d'atomes de deutérium dans la molécule de this compound. Cela peut être réalisé par diverses méthodes, y compris l'utilisation de réactifs ou de solvants deutérés lors du processus de synthèse . Les conditions de réaction impliquent généralement l'utilisation de solvants deutérés tels que le diméthylsulfoxyde deutéré (DMSO) ou l'éthanol deutéré, et les réactions sont réalisées sous des températures et des pressions contrôlées pour assurer l'incorporation d'atomes de deutérium .
Méthodes de Production Industrielle: : La production industrielle de this compound D6 suit des voies de synthèse similaires, mais à plus grande échelle. Le processus implique l'utilisation de réacteurs automatisés et un contrôle précis des conditions de réaction pour garantir un rendement élevé et la pureté du produit final .
Analyse Des Réactions Chimiques
Types de Réactions: : Le Proguanil D6 subit diverses réactions chimiques, notamment :
Oxydation: Le this compound D6 peut être oxydé pour former son métabolite actif, le cycloguanil.
Réduction: Les réactions de réduction peuvent convertir le this compound D6 en son composé parent, le this compound.
Substitution: Des réactions de substitution peuvent se produire au niveau du groupe chloro, conduisant à la formation de différents dérivés.
Réactifs et Conditions Courantes
Substitution: Les réactions de substitution impliquent souvent des nucléophiles tels que les amines ou les thiols en milieu basique.
Principaux Produits
Cycloguanil: Le principal produit formé par l'oxydation du this compound D6.
Divers Dérivés: Formés par des réactions de substitution.
Applications de la Recherche Scientifique
Le this compound D6 a une large gamme d'applications dans la recherche scientifique, notamment :
Médecine: En cours d'investigation pour ses propriétés anticancéreuses potentielles, en particulier dans le cancer du sein et de la vessie
Mécanisme d'Action
Le this compound D6 exerce ses effets en inhibant l'enzyme dihydrofolate réductase, qui est essentielle à la reproduction du parasite du paludisme . Cette inhibition empêche le parasite de synthétiser l'ADN et de se répliquer, stoppant ainsi l'infection . Le marquage au deutérium dans le this compound D6 permet un suivi plus précis de la distribution et du métabolisme du médicament dans l'organisme .
Comparaison Avec Des Composés Similaires
Composés Similaires
Proguanil: Le composé parent du this compound D6, utilisé pour la prophylaxie et le traitement du paludisme.
Cycloguanil: Le métabolite actif du this compound, formé par oxydation.
Chloroquine: Un autre médicament antipaludique souvent utilisé en association avec le this compound.
Unicité: : Le this compound D6 est unique en raison de son marquage au deutérium, qui améliore sa stabilité et permet des études pharmacocinétiques plus précises que ses homologues non deutérés . Cela en fait un outil précieux dans la recherche scientifique et le développement de médicaments.
Propriétés
| Proguanil inhibits the dihydrofolate reductase of plasmodia and thereby blocks the biosynthesis of purines and pyrimidines, which are essential for DNA synthesis and cell multiplication. This leads to failure of nuclear division at the time of schizont formation in erythrocytes and liver. | |
Numéro CAS |
500-92-5 |
Formule moléculaire |
C11H16ClN5 |
Poids moléculaire |
259.77 g/mol |
Nom IUPAC |
1-[amino-(4-chloroanilino)methylidene]-2-(1,1,1,3,3,3-hexadeuteriopropan-2-yl)guanidine |
InChI |
InChI=1S/C11H16ClN5/c1-7(2)15-10(13)17-11(14)16-9-5-3-8(12)4-6-9/h3-7H,1-2H3,(H5,13,14,15,16,17)/i1D3,2D3 |
Clé InChI |
SSOLNOMRVKKSON-WFGJKAKNSA-N |
SMILES |
CC(C)N=C(N)N=C(N)NC1=CC=C(C=C1)Cl |
SMILES isomérique |
[2H]C([2H])([2H])C(C([2H])([2H])[2H])N=C(N)N=C(N)NC1=CC=C(C=C1)Cl |
SMILES canonique |
CC(C)N=C(N)N=C(N)NC1=CC=C(C=C1)Cl |
melting_point |
129 °C |
| 500-92-5 | |
Description physique |
Solid |
Pureté |
> 95% |
Quantité |
Milligrams-Grams |
Numéros CAS associés |
637-32-1 (hydrochloride) |
Solubilité |
2.86e-01 g/L |
Synonymes |
Bigumal Chlorguanid Chloriguane Chloroguanide Chloroguanide Hydrochloride Hydrochloride, Chloroguanide Hydrochloride, Proguanil Paludrin Paludrine Proguanil Proguanil Hydrochloride |
Origine du produit |
United States |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: How does Proguanil exert its antimalarial effect?
A1: this compound itself has weak antimalarial activity. Its effectiveness stems from its active metabolite, Cycloguanil, a potent inhibitor of dihydrofolate reductase (DHFR) []. DHFR is a crucial enzyme in the folate metabolic pathway, essential for DNA synthesis and cellular replication in parasites like Plasmodium falciparum []. By inhibiting DHFR, Cycloguanil disrupts DNA synthesis and ultimately kills the parasite [, ].
Q2: Are there other mechanisms by which this compound impacts Plasmodium falciparum?
A2: Research suggests this compound, in combination with Atovaquone, might interfere with mitochondrial electron transport and collapse mitochondrial membrane potential in the parasite, further contributing to its antimalarial activity [].
Q3: Does this compound affect other stages of the Plasmodium life cycle besides the erythrocytic stage?
A3: Yes, both this compound and Atovaquone demonstrate activity against gametocytes and pre-erythrocytic (hepatic) stages of malaria parasites []. This is supported by studies indicating that short-term this compound administration might provide causal prophylaxis for Plasmodium vivax by inhibiting liver-stage schizonts, although it doesn't seem to prevent late attacks related to hypnozoite reactivation [].
Q4: How is this compound metabolized in the human body?
A4: this compound is primarily metabolized in the liver by cytochrome P450 (CYP) enzymes, specifically CYP2C19 and CYP3A4 [, ]. The primary metabolic pathway involves CYP2C19-mediated conversion to its active metabolite, Cycloguanil [, ].
Q5: What factors contribute to the variability in this compound metabolism among individuals?
A5: Inter-individual variability in this compound metabolism is influenced by several factors, primarily genetic polymorphisms in the CYP2C19 gene [, ]. Individuals homozygous for the CYP2C19*2 allele exhibit significantly reduced metabolic capacity, leading to higher this compound and lower Cycloguanil levels []. Other factors include co-administration of drugs that are CYP2C19 inducers or inhibitors [], and variations in the expression and activity of other enzymes involved in this compound metabolism, like CYP3A4 [].
Q6: How is this compound eliminated from the body?
A6: Both this compound and Cycloguanil are predominantly eliminated through the kidneys []. Therefore, dosage adjustments are necessary for patients with renal impairment to prevent drug accumulation [].
Q7: Are there documented cases of resistance to this compound?
A7: Yes, this compound resistance has been observed and is primarily attributed to point mutations in the dihydrofolate reductase (DHFR) gene of Plasmodium falciparum [, ]. The S108N mutation is particularly associated with this compound resistance [, ].
Q8: Is there cross-resistance between this compound and other antimalarial drugs?
A8: Yes, cross-resistance has been observed between this compound and Pyrimethamine, another antifolate drug []. This is attributed to their shared mechanism of action, both targeting the DHFR enzyme in the parasite. The presence of the triple mutant DHFR haplotype (S108N+N51I+C59N) in Plasmodium falciparum has been linked to resistance to both drugs [, ].
Q9: Does this compound interact with other drugs?
A9: Yes, this compound's metabolism can be affected by co-administration with other drugs metabolized by CYP2C19, such as Phenytoin []. Concomitant use of Phenytoin, a CYP2C19 inducer, can decrease this compound's area under the curve (AUC) and maximum concentration (Cmax), potentially impacting its efficacy [].
Q10: Beyond malaria, are there other potential therapeutic applications for this compound?
A10: Emerging research suggests that this compound may have anti-cancer properties, particularly in breast cancer. Studies have shown that this compound inhibits the growth of breast cancer cells in vitro and in vivo, potentially by inducing oxidative stress, disrupting mitochondrial function, and triggering apoptosis [, ].
Q11: What are the key considerations in formulating this compound for therapeutic use?
A11: this compound formulations aim to optimize solubility, bioavailability, and stability []. The choice of excipients and manufacturing processes can significantly influence these factors. For instance, some herbal formulations may significantly impact the dissolution profile of this compound tablets, potentially altering its bioavailability and warranting further investigation for potential herb-drug interactions [].
Q12: What analytical techniques are commonly employed to quantify this compound and its metabolites?
A12: High-performance liquid chromatography (HPLC) is widely used to measure this compound and its metabolites in biological samples like plasma and urine [, , ]. Ultra-performance liquid chromatography (UPLC) offers enhanced speed and sensitivity for pharmacokinetic studies [].
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.


![(6R,7R)-7-[(2,2-dimethyl-1-oxopropyl)amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid](/img/structure/B194008.png)
![(6R,7R)-7-[[(2R)-2-amino-2-phenylacetyl]amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-3-ene-2-carboxylic acid](/img/structure/B194010.png)