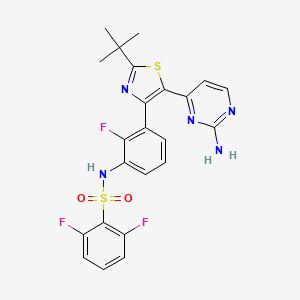
Dabrafénib
Vue d'ensemble
Description
Dabrafenib est un inhibiteur de kinase principalement utilisé dans le traitement des cancers associés à des mutations du gène BRAF, tels que le mélanome, le cancer du poumon non à petites cellules et le cancer de la thyroïde . Il est commercialisé sous le nom de marque Tafinlar et a été approuvé pour la première fois pour un usage médical aux États-Unis en mai 2013 .
Applications De Recherche Scientifique
Dabrafenib has a wide range of scientific research applications, particularly in the fields of oncology and pharmacology. It is used to study the effects of BRAF mutations on cell proliferation and tumor growth . In combination with trametinib, dabrafenib has been shown to improve response rates and progression-free survival in patients with BRAF V600E-mutant cancers . Additionally, dabrafenib is used in preclinical studies to investigate its potential in treating other types of cancer, such as colorectal and non-small cell lung cancers .
Mécanisme D'action
Target of Action
Dabrafenib is a potent and selective inhibitor of some mutated forms of the protein kinase B-raf (BRAF) . The primary target of Dabrafenib is the BRAF V600E mutation, which is a serine/threonine protein kinase involved in cell growth .
Mode of Action
Dabrafenib works by binding to the ATP pocket of the BRAF enzyme, thereby inhibiting its activity . This inhibition is competitive and selective, with Dabrafenib showing a higher affinity for mutant forms of BRAF, including BRAF V600E, BRAF V600K, and BRAF V600D .
Biochemical Pathways
The primary biochemical pathway affected by Dabrafenib is the mitogen-activated protein kinase (MAPK) pathway . The BRAF mutations result in the activation of the MAPK pathway, which may promote tumor cell growth . By inhibiting BRAF, Dabrafenib disrupts this pathway, leading to a decrease in tumor cell proliferation .
Pharmacokinetics
The metabolism of Dabrafenib is primarily mediated by CYP2C8 and CYP3A4 to form hydroxy-dabrafenib . Hydroxy-dabrafenib is further oxidized via CYP3A4 to form carboxy-dabrafenib, which is subsequently excreted in bile and urine . Carboxy-dabrafenib is decarboxylated to form desmethyl-dabrafenib, which may be reabsorbed from the gut .
Result of Action
The molecular and cellular effects of Dabrafenib’s action include the regression and decreased proliferation of cells . By inhibiting the MAPK pathway in BRAF mutated cells, Dabrafenib leads to a decrease in tumor cell growth . This results in the regression of the cells and a decrease in their proliferation .
Action Environment
The action, efficacy, and stability of Dabrafenib can be influenced by various environmental factors. For instance, the presence of other drugs can affect the metabolism of Dabrafenib, potentially altering its efficacy . Additionally, factors such as the patient’s overall health, the presence of other diseases, and individual genetic factors can also influence the action of Dabrafenib .
Analyse Biochimique
Biochemical Properties
Dabrafenib acts as a competitive and selective BRAF inhibitor by binding to its ATP pocket . It has a higher affinity for mutant forms of BRAF, including BRAF V600E, BRAF V600K, and BRAF V600D . BRAF is a serine/threonine protein kinase and is involved in activating the RAS/MAPK pathway .
Cellular Effects
Dabrafenib has multifaceted influences on cells, particularly on migration at concentrations of 10 and 25 μM . It induces a decrease in cell viability, impedes cellular adhesion to the matrix, inhibits cellular aggregation and spheroid formation, arrests the cell cycle in the G1 phase, and induces apoptosis .
Molecular Mechanism
Dabrafenib inhibits the MAPK pathway by targeting the BRAF V600E mutation . This inhibition results in decreased MEK and ERK phosphorylation and inhibition of cell proliferation .
Temporal Effects in Laboratory Settings
In a BRAF V600E-containing xenograft model of human melanoma, orally administered dabrafenib inhibited ERK activation, downregulated Ki67, and upregulated p27, leading to tumor growth inhibition . Dabrafenib also induced MAPK pathway activation in wild-type BRAF cells through CRAF (RAF1) signaling .
Dosage Effects in Animal Models
In a clinically relevant multidose cisplatin mouse model, dabrafenib given orally twice daily with cisplatin showed protective effects as determined by functional hearing tests and cochlear outer hair cell counts . A dabrafenib dose of 3 mg/kg BW, twice daily, in mice, was determined to be the minimum effective dose .
Metabolic Pathways
The main elimination route of dabrafenib is the oxidative metabolism via CYP3A4/2C8 and biliary excretion . Among the three major metabolites identified, hydroxy-dabrafenib appears to contribute to the pharmacological activity .
Transport and Distribution
Dabrafenib is almost completely absorbed after administration of the recommended dose of 150 mg twice daily . It shows a time-dependent increase in apparent clearance following multiple doses, which is likely due to induction of its own metabolism through cytochrome P450 (CYP) 3A4 .
Subcellular Localization
The distinctive subcellular localization of PCTAIRE CDKs in the cytoplasm and at the plasma membrane, often in association with cytoskeletal elements, suggests roles in vesicular transport, adhesion, and cell motility
Méthodes De Préparation
La synthèse de dabrafenib implique plusieurs étapes clés, notamment la sulfamidation, l'halogénation, la cyclisation thiazolique, l'acylation et la cyclisation pyrimidinique . Le processus commence par le 3-(3-amino-2-fluorophényl)-3-oxa-propionate comme matière de départ. Ce composé subit une série de réactions pour former le produit final, le dabrafenib . Les méthodes de production industrielle sont conçues pour être concises et douces, ce qui les rend appropriées pour la fabrication à grande échelle .
Analyse Des Réactions Chimiques
Dabrafenib subit diverses réactions chimiques, impliquant principalement un métabolisme oxydatif via les enzymes du cytochrome P450 CYP3A4 et CYP2C8 . Les principaux métabolites formés comprennent l'hydroxy-dabrafenib, qui contribue à l'activité pharmacologique du composé . Les réactifs couramment utilisés dans ces réactions comprennent la formamide et diverses bases . Les principaux produits de ces réactions sont les métabolites qui sont excrétés dans la bile et l'urine .
Applications de la recherche scientifique
Dabrafenib a un large éventail d'applications en recherche scientifique, en particulier dans les domaines de l'oncologie et de la pharmacologie. Il est utilisé pour étudier les effets des mutations BRAF sur la prolifération cellulaire et la croissance tumorale . En association avec le trametinib, le dabrafenib a montré qu'il améliorait les taux de réponse et la survie sans progression chez les patients atteints de cancers porteurs de la mutation BRAF V600E . En outre, le dabrafenib est utilisé dans des études précliniques pour étudier son potentiel dans le traitement d'autres types de cancer, tels que les cancers colorectaux et les cancers du poumon non à petites cellules .
Mécanisme d'action
Dabrafenib est un inhibiteur compétitif et sélectif de la kinase BRAF, ciblant spécifiquement la poche ATP de l'enzyme . Il a une affinité plus élevée pour les formes mutantes de BRAF, y compris BRAF V600E, BRAF V600K et BRAF V600D . En inhibant BRAF, le dabrafenib perturbe la voie de signalisation MAPK, entraînant l'arrêt du cycle cellulaire et l'apoptose dans les cellules cancéreuses . Ce mécanisme d'action rend le dabrafenib efficace dans le traitement des cancers induits par des mutations BRAF .
Comparaison Avec Des Composés Similaires
Dabrafenib est souvent comparé à d'autres inhibiteurs de BRAF, tels que le vemurafenib et l'encorafenib . Bien que les trois composés ciblent la kinase BRAF, le dabrafenib a une structure chimique unique qui permet une affinité et une sélectivité de liaison différentes . Cette unicité contribue à ses profils pharmacocinétiques et pharmacodynamiques distincts . D'autres composés similaires comprennent le trametinib, qui est souvent utilisé en association avec le dabrafenib pour améliorer ses effets thérapeutiques .
Propriétés
IUPAC Name |
N-[3-[5-(2-aminopyrimidin-4-yl)-2-tert-butyl-1,3-thiazol-4-yl]-2-fluorophenyl]-2,6-difluorobenzenesulfonamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C23H20F3N5O2S2/c1-23(2,3)21-30-18(19(34-21)16-10-11-28-22(27)29-16)12-6-4-9-15(17(12)26)31-35(32,33)20-13(24)7-5-8-14(20)25/h4-11,31H,1-3H3,(H2,27,28,29) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
BFSMGDJOXZAERB-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)(C)C1=NC(=C(S1)C2=NC(=NC=C2)N)C3=C(C(=CC=C3)NS(=O)(=O)C4=C(C=CC=C4F)F)F | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C23H20F3N5O2S2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID20152499 | |
| Record name | Dabrafenib | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID20152499 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
519.6 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
very slightly soluble at pH 1 | |
| Record name | Dabrafenib | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08912 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Mechanism of Action |
Dabrafenib is a competitive and selective BRAF inhibitor by binding to its ATP pocket.. Although dabrafenib can inhibit wild-type BRAF, it has a higher affinity for mutant forms of BRAF, including BRAF V600E, BRAF V600K, and BRAF V600D. BRAF is a serine/threonine protein kinase and is involved in activating the RAS/RAF/MEK/ERK or MAPK pathway, a pathway that is implicated in cell cycle progression, cell proliferation, and arresting apoptosis.Therefore, constitutive active mutation of BRAF such as BRAF V600E is frequently observed in many types of cancer, including melanoma, lung cancer, and colon cancer. | |
| Record name | Dabrafenib | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08912 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
1195765-45-7 | |
| Record name | Dabrafenib | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=1195765-45-7 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Dabrafenib [USAN:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=1195765457 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Dabrafenib | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08912 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Dabrafenib | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID20152499 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | N-[3-[5-(2-aminopyrimidin-4-yl)-2-tert-butyl-1,3-thiazol-4-yl]-2-fluorophenyl]-2,6-difluorobenzenesulfonamide | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.215.965 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | DABRAFENIB | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/QGP4HA4G1B | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Synthesis routes and methods I
Procedure details





Synthesis routes and methods II
Procedure details





Synthesis routes and methods III
Procedure details






Q1: What is the primary molecular target of Dabrafenib?
A1: Dabrafenib is a potent ATP-competitive inhibitor specifically targeting the V600 mutant B-Raf kinase (BRAF), primarily BRAF V600E and to a lesser extent BRAF V600K. [, , , ]
Q2: How does Dabrafenib's inhibition of BRAF affect downstream signaling?
A2: Dabrafenib binding to BRAF prevents the phosphorylation and activation of downstream MEK proteins (MEK1 and MEK2), effectively inhibiting the RAS/RAF/MEK/ERK (MAPK) signaling pathway. This pathway is crucial for cell division, differentiation, and various developmental processes. [, , ]
Q3: What is the molecular formula and weight of Dabrafenib?
A3: The provided research papers do not explicitly state the molecular formula and weight of Dabrafenib. Please refer to drug information resources like PubChem or Drugbank for this information.
Q4: Is there any spectroscopic data available for Dabrafenib in the research?
A4: The research papers do not provide detailed spectroscopic data for Dabrafenib.
Q5: How does light exposure affect Dabrafenib stability?
A5: Dabrafenib exhibits photosensitivity in both organic and aqueous solutions, leading to its degradation and the formation of a novel fluorescent derivative, dabrafenib_photo 2. []
Q6: What are the implications of Dabrafenib's photosensitivity?
A6: Researchers handling Dabrafenib should be aware of its photosensitivity and take precautions during storage and experiments to minimize light exposure. This includes using amber vials for storage and performing experiments under controlled lighting conditions. []
Q7: Does Dabrafenib exhibit any catalytic properties itself?
A7: Dabrafenib is not described as possessing intrinsic catalytic properties. It functions as an enzyme inhibitor, specifically targeting BRAF kinase.
Q8: Has computational chemistry been used to study Dabrafenib?
A8: The provided research papers do not extensively discuss computational chemistry studies specific to Dabrafenib.
Q9: What is known about the Structure-Activity Relationship (SAR) of Dabrafenib?
A9: While specific SAR studies are not detailed in the research, it's understood that modifications impacting Dabrafenib's interaction with the ATP-binding site of BRAF V600E/K will alter its potency and selectivity. [, ]
Q10: What is the recommended storage for Dabrafenib?
A10: Given its photosensitivity, Dabrafenib should be stored in amber vials to protect from light degradation. Specific temperature and storage conditions would be outlined in the manufacturer's information. []
Q11: Do the provided research papers discuss SHE regulations related to Dabrafenib?
A11: The research papers primarily focus on Dabrafenib's biological activity and clinical effects and do not delve into SHE regulations.
Q12: How is Dabrafenib metabolized in the body?
A12: Dabrafenib is primarily metabolized by CYP2C8 (56%-67%) and CYP3A4 (24%) enzymes in the liver. []
Q13: Are there any known drug-drug interactions (DDIs) with Dabrafenib?
A13: Dabrafenib is a potential inducer of CYP3A4 and can be a victim of both CYP3A4 and CYP2C8 inhibitors. Co-administration with strong inhibitors of these enzymes, like ketoconazole (CYP3A4) or gemfibrozil (CYP2C8), can significantly increase Dabrafenib exposure. [, ]
Q14: Does Dabrafenib affect the metabolism of other drugs?
A14: Yes, Dabrafenib has shown inhibition of several CYP enzymes in vitro, including CYP2C8, 2C9, 2C19, 3A4. Notably, it can decrease the AUC of S-warfarin, a CYP2C9 substrate, indicating a potential for interaction. [, ]
Q15: What is the clinical significance of Dabrafenib's interaction with CYP enzymes?
A15: Clinicians should be cautious when co-prescribing Dabrafenib with drugs metabolized by CYP2C8 and CYP3A4, as dose adjustments may be necessary to avoid adverse events or reduced efficacy. Monitoring for potential drug interactions is essential. [, , ]
Q16: Which human melanoma cell lines have been used to study Dabrafenib's efficacy in vitro?
A16: Several human melanoma cell lines, including A375, 397, 624.38, WM3918, WM9838BR, WM983B, and DB-1, have been used in various studies to assess Dabrafenib's impact on cell signaling, proliferation, and response to treatment. [, , ]
Q17: What in vivo models have been used to study Dabrafenib?
A17: Mouse xenograft models implanted with human melanoma cells have been used to assess Dabrafenib's efficacy in vivo, allowing for tumor growth monitoring and assessment of therapeutic response. []
Q18: What are the key findings from clinical trials evaluating Dabrafenib?
A18: Clinical trials have demonstrated that Dabrafenib, as monotherapy or combined with Trametinib (a MEK inhibitor), significantly improves progression-free survival and overall survival in patients with BRAF V600E/K-mutated melanoma. [, , , , ]
Q19: What are the mechanisms of resistance to Dabrafenib?
A19: One of the primary resistance mechanisms to Dabrafenib involves the reactivation of the MAPK pathway, often through the development of new mutations in NRAS or KRAS, or through upregulation of growth factor receptors like EGFR. [, , ]
Q20: Is there cross-resistance between Dabrafenib and other BRAF inhibitors?
A20: Cross-resistance between Dabrafenib and other BRAF inhibitors, such as Vemurafenib, can occur. This is often due to shared resistance mechanisms involving reactivation of the MAPK pathway or other signaling pathways that bypass BRAF inhibition. []
Q21: What are the common cutaneous side effects observed with Dabrafenib?
A21: Common cutaneous side effects include papillomas, palmoplantar hyperkeratosis, alopecia, and seborrheic dermatitis-like eruption. In some cases, development of keratoacanthoma-like squamous cell carcinoma has been reported. []
Q22: Are there any specific drug delivery strategies being explored for Dabrafenib?
A22: The research papers provided do not delve into specific drug delivery strategies being investigated for Dabrafenib.
Q23: What is the primary biomarker for selecting patients for Dabrafenib treatment?
A23: The presence of BRAF V600E or V600K mutations in tumor tissue is the primary biomarker for identifying patients who are eligible for treatment with Dabrafenib. [, , , ]
Q24: Are there any biomarkers being investigated to monitor response to Dabrafenib treatment?
A24: Research suggests that circulating levels of the PTTG1 protein might serve as a potential biomarker for monitoring patient response to targeted therapy, including Dabrafenib. []
Q25: What analytical techniques are commonly used to quantify Dabrafenib and its metabolites?
A25: Liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) is commonly employed for quantifying Dabrafenib and its metabolites in biological samples. [, ]
Q26: Do the provided research papers discuss the environmental impact of Dabrafenib?
A26: The environmental impact and degradation of Dabrafenib are not discussed in the provided research papers.
Q27: Are there any studies on the dissolution and solubility of Dabrafenib?
A27: The provided research papers do not specifically address the dissolution and solubility characteristics of Dabrafenib.
Q28: What are the key parameters assessed during the validation of analytical methods for Dabrafenib?
A28: While not explicitly detailed, validation of analytical methods like LC-MS/MS for Dabrafenib quantification would involve assessing accuracy, precision, specificity, linearity, range, limit of detection, limit of quantification, and robustness. [, , ]
Q29: Do the research papers discuss specific quality control measures for Dabrafenib?
A29: The research papers focus on scientific aspects and do not delve into detailed quality control measures employed during Dabrafenib development and manufacturing.
Q30: Does Dabrafenib have any known impact on the immune system?
A30: Dabrafenib, while not directly targeting the immune system, has been shown to impact the tumor microenvironment. Research suggests it can modulate the differentiation and function of myeloid-derived suppressor cells (MDSCs), which play a role in immune suppression within tumors. [, ]
Q31: Are there any known interactions between Dabrafenib and drug transporters?
A31: Specific information regarding interactions between Dabrafenib and drug transporters is not provided in the research papers.
Q32: Does Dabrafenib induce or inhibit drug-metabolizing enzymes?
A32: Dabrafenib has been shown to both inhibit and induce drug-metabolizing enzymes. It inhibits various CYP enzymes (CYP2C8, 2C9, 2C19, 3A4), potentially affecting the metabolism of co-administered drugs. Conversely, it can also induce CYP3A4, leading to potential alterations in its own metabolism and that of other CYP3A4 substrates. [, , ]
Q33: Is there information on Dabrafenib's biocompatibility and biodegradability?
A33: The provided research papers do not focus on Dabrafenib's biocompatibility or biodegradability aspects.
Q34: What are alternative treatment options for BRAF V600E/K-mutated melanoma?
A34: Other BRAF inhibitors like Vemurafenib, as well as MEK inhibitors like Trametinib and Cobimetinib, are alternative treatment options for BRAF V600E/K-mutated melanoma. Immunotherapy options, including immune checkpoint inhibitors like Nivolumab and Ipilimumab, are also used in the treatment of advanced melanoma. [, , , ]
Q35: Do the research papers mention strategies for recycling or managing Dabrafenib waste?
A35: The research papers do not address strategies for recycling or managing Dabrafenib waste.
Q36: What research infrastructure and resources are important for studying Dabrafenib?
A36: Important resources include access to well-characterized human melanoma cell lines, in vivo models like mouse xenografts, high-throughput screening platforms for evaluating drug efficacy and potential resistance mechanisms, and advanced analytical techniques like LC-MS/MS for quantifying drug concentrations and monitoring metabolic activity.
Q37: When was Dabrafenib approved for the treatment of melanoma?
A37: Dabrafenib received FDA approval for the treatment of metastatic melanoma harboring the BRAF V600E mutation in 2013. [, ]
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.


![4-(Tert-butyl)-N-(6-hydroxy-5-(2-methoxyphenoxy)-[2,2'-bipyrimidin]-4-yl)benzenesulfonamide](/img/structure/B601010.png)
