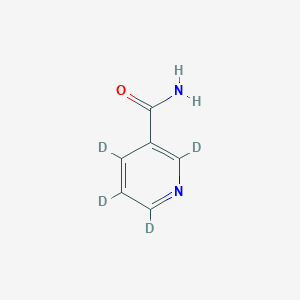
Nicotinamide-d4
Numéro de catalogue B132304
Poids moléculaire: 126.15 g/mol
Clé InChI: DFPAKSUCGFBDDF-RHQRLBAQSA-N
Attention: Uniquement pour un usage de recherche. Non destiné à un usage humain ou vétérinaire.
Patent
US07737158B2
Procedure details


High performance liquid chromatography was used to detect Nampt reaction products. HPLC was performed with Waters 515 pumps and a 2487 detector (Waters, Mass.) with a Supelco LC-18-T column (15 cm×4.6 cm; Supelco, Pa.). The Nampt reaction was conducted at 37° C. for 15 min in 500 μl of reaction buffer (50 mM Tris-HCl [pH 7.5], 10 mM MgCl2, 50 mM nicotinamide, 0.2 mM PRPP) with 50 μg of the recombinant Nampt protein. The reaction was terminated by adding 125 μl of 1 M HClO4. Protein was then precipitated at 18,000 g, and 500 μl of the supernatant was neutralized with 40 μl of 3 M K2CO3. After centrifugation, 100 μl of sample was mixed with 400 μl of Buffer A (50 mM K2PO4/KHPO4, pH 7.0) and loaded into the HPLC system. The products from Nampt reaction were monitored by absorbance at 261 nm. Results of HPLC detection of Nampt reaction products showed that the mouse Nampt produced nicotinamide mononucleotide (NMN) from nicotinamide and PRPP (see, e.g., FIG. 4D). Nampt failed to catalyze the synthesis of nicotinic acid mononucleotide (NaMN) from nicotinic acid and PRPP (see, e.g., FIGS. 13A and 13B), confirming the substrate specificity of this enzyme. In isolated reactions, it was also confirmed that Nmnat catalyzed the synthesis of NAD from NMN and ATP.
Name
Tris-HCl
Quantity
0 (± 1) mol
Type
reactant
Reaction Step One





Name

Name
Identifiers


|
REACTION_CXSMILES
|
C(O)C(N)(CO)CO.Cl.[Mg+2].[Cl-].[Cl-].[C:13]([NH2:21])(=[O:20])[C:14]1[CH:19]=[CH:18][CH:17]=[N:16][CH:15]=1.[CH2:22]([O:39][P:40]([OH:43])([OH:42])=[O:41])[C@H:23]1[O:27][C@H:26]([O:28][P:29]([O:32][P:33]([OH:36])([OH:35])=[O:34])([OH:31])=[O:30])[C@H:25]([OH:37])[C@@H:24]1[OH:38]>>[CH:18]1[CH:17]=[N+:16]([CH:26]2[O:27][C@H:23]([CH2:22][O:39][P:40]([O-:43])([OH:42])=[O:41])[C@@H:24]([OH:38])[C@H:25]2[OH:37])[CH:15]=[C:14]([C:13]([NH2:21])=[O:20])[CH:19]=1.[C:13]([NH2:21])(=[O:20])[C:14]1[CH:19]=[CH:18][CH:17]=[N:16][CH:15]=1.[CH2:22]([O:39][P:40]([OH:43])([OH:42])=[O:41])[C@H:23]1[O:27][C@H:26]([O:28][P:29]([O:32][P:33]([OH:36])([OH:35])=[O:34])([OH:31])=[O:30])[C@H:25]([OH:37])[C@@H:24]1[OH:38] |f:0.1,2.3.4|
|
Inputs


Step One
|
Name
|
Tris-HCl
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C(CO)(CO)N)O.Cl
|
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
[Mg+2].[Cl-].[Cl-]
|
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)N
|
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C([C@@H]1[C@H]([C@H]([C@H](O1)OP(=O)(O)OP(=O)(O)O)O)O)OP(=O)(O)O
|
Conditions


Other
|
Conditions are dynamic
|
1
|
|
Details
|
See reaction.notes.procedure_details.
|
Workups


CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
Nampt reaction products
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
The Nampt reaction
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
The reaction was terminated
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
by adding 125 μl of 1 M HClO4
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
Protein was then precipitated at 18,000 g, and 500 μl of the supernatant
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
After centrifugation, 100 μl of sample was mixed with 400 μl of Buffer A (50 mM K2PO4/KHPO4, pH 7.0)
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
The products from Nampt reaction
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
Results of HPLC detection of Nampt reaction products
|
Outcomes


Product
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
C1=CC(=C[N+](=C1)C2[C@@H]([C@@H]([C@H](O2)COP(=O)(O)[O-])O)O)C(=O)N
|
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)N
|
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
C([C@@H]1[C@H]([C@H]([C@H](O1)OP(=O)(O)OP(=O)(O)O)O)O)OP(=O)(O)O
|
Source


|
Source
|
Open Reaction Database (ORD) |
|
Description
|
The Open Reaction Database (ORD) is an open-access schema and infrastructure for structuring and sharing organic reaction data, including a centralized data repository. The ORD schema supports conventional and emerging technologies, from benchtop reactions to automated high-throughput experiments and flow chemistry. Our vision is that a consistent data representation and infrastructure to support data sharing will enable downstream applications that will greatly improve the state of the art with respect to computer-aided synthesis planning, reaction prediction, and other predictive chemistry tasks. |
Patent
US07737158B2
Procedure details


High performance liquid chromatography was used to detect Nampt reaction products. HPLC was performed with Waters 515 pumps and a 2487 detector (Waters, Mass.) with a Supelco LC-18-T column (15 cm×4.6 cm; Supelco, Pa.). The Nampt reaction was conducted at 37° C. for 15 min in 500 μl of reaction buffer (50 mM Tris-HCl [pH 7.5], 10 mM MgCl2, 50 mM nicotinamide, 0.2 mM PRPP) with 50 μg of the recombinant Nampt protein. The reaction was terminated by adding 125 μl of 1 M HClO4. Protein was then precipitated at 18,000 g, and 500 μl of the supernatant was neutralized with 40 μl of 3 M K2CO3. After centrifugation, 100 μl of sample was mixed with 400 μl of Buffer A (50 mM K2PO4/KHPO4, pH 7.0) and loaded into the HPLC system. The products from Nampt reaction were monitored by absorbance at 261 nm. Results of HPLC detection of Nampt reaction products showed that the mouse Nampt produced nicotinamide mononucleotide (NMN) from nicotinamide and PRPP (see, e.g., FIG. 4D). Nampt failed to catalyze the synthesis of nicotinic acid mononucleotide (NaMN) from nicotinic acid and PRPP (see, e.g., FIGS. 13A and 13B), confirming the substrate specificity of this enzyme. In isolated reactions, it was also confirmed that Nmnat catalyzed the synthesis of NAD from NMN and ATP.
Name
Tris-HCl
Quantity
0 (± 1) mol
Type
reactant
Reaction Step One





Name

Name
Identifiers


|
REACTION_CXSMILES
|
C(O)C(N)(CO)CO.Cl.[Mg+2].[Cl-].[Cl-].[C:13]([NH2:21])(=[O:20])[C:14]1[CH:19]=[CH:18][CH:17]=[N:16][CH:15]=1.[CH2:22]([O:39][P:40]([OH:43])([OH:42])=[O:41])[C@H:23]1[O:27][C@H:26]([O:28][P:29]([O:32][P:33]([OH:36])([OH:35])=[O:34])([OH:31])=[O:30])[C@H:25]([OH:37])[C@@H:24]1[OH:38]>>[CH:18]1[CH:17]=[N+:16]([CH:26]2[O:27][C@H:23]([CH2:22][O:39][P:40]([O-:43])([OH:42])=[O:41])[C@@H:24]([OH:38])[C@H:25]2[OH:37])[CH:15]=[C:14]([C:13]([NH2:21])=[O:20])[CH:19]=1.[C:13]([NH2:21])(=[O:20])[C:14]1[CH:19]=[CH:18][CH:17]=[N:16][CH:15]=1.[CH2:22]([O:39][P:40]([OH:43])([OH:42])=[O:41])[C@H:23]1[O:27][C@H:26]([O:28][P:29]([O:32][P:33]([OH:36])([OH:35])=[O:34])([OH:31])=[O:30])[C@H:25]([OH:37])[C@@H:24]1[OH:38] |f:0.1,2.3.4|
|
Inputs


Step One
|
Name
|
Tris-HCl
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C(CO)(CO)N)O.Cl
|
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
[Mg+2].[Cl-].[Cl-]
|
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)N
|
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C([C@@H]1[C@H]([C@H]([C@H](O1)OP(=O)(O)OP(=O)(O)O)O)O)OP(=O)(O)O
|
Conditions


Other
|
Conditions are dynamic
|
1
|
|
Details
|
See reaction.notes.procedure_details.
|
Workups


CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
Nampt reaction products
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
The Nampt reaction
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
The reaction was terminated
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
by adding 125 μl of 1 M HClO4
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
Protein was then precipitated at 18,000 g, and 500 μl of the supernatant
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
After centrifugation, 100 μl of sample was mixed with 400 μl of Buffer A (50 mM K2PO4/KHPO4, pH 7.0)
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
The products from Nampt reaction
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
Results of HPLC detection of Nampt reaction products
|
Outcomes


Product
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
C1=CC(=C[N+](=C1)C2[C@@H]([C@@H]([C@H](O2)COP(=O)(O)[O-])O)O)C(=O)N
|
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)N
|
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
C([C@@H]1[C@H]([C@H]([C@H](O1)OP(=O)(O)OP(=O)(O)O)O)O)OP(=O)(O)O
|
Source


|
Source
|
Open Reaction Database (ORD) |
|
Description
|
The Open Reaction Database (ORD) is an open-access schema and infrastructure for structuring and sharing organic reaction data, including a centralized data repository. The ORD schema supports conventional and emerging technologies, from benchtop reactions to automated high-throughput experiments and flow chemistry. Our vision is that a consistent data representation and infrastructure to support data sharing will enable downstream applications that will greatly improve the state of the art with respect to computer-aided synthesis planning, reaction prediction, and other predictive chemistry tasks. |
Patent
US07737158B2
Procedure details


High performance liquid chromatography was used to detect Nampt reaction products. HPLC was performed with Waters 515 pumps and a 2487 detector (Waters, Mass.) with a Supelco LC-18-T column (15 cm×4.6 cm; Supelco, Pa.). The Nampt reaction was conducted at 37° C. for 15 min in 500 μl of reaction buffer (50 mM Tris-HCl [pH 7.5], 10 mM MgCl2, 50 mM nicotinamide, 0.2 mM PRPP) with 50 μg of the recombinant Nampt protein. The reaction was terminated by adding 125 μl of 1 M HClO4. Protein was then precipitated at 18,000 g, and 500 μl of the supernatant was neutralized with 40 μl of 3 M K2CO3. After centrifugation, 100 μl of sample was mixed with 400 μl of Buffer A (50 mM K2PO4/KHPO4, pH 7.0) and loaded into the HPLC system. The products from Nampt reaction were monitored by absorbance at 261 nm. Results of HPLC detection of Nampt reaction products showed that the mouse Nampt produced nicotinamide mononucleotide (NMN) from nicotinamide and PRPP (see, e.g., FIG. 4D). Nampt failed to catalyze the synthesis of nicotinic acid mononucleotide (NaMN) from nicotinic acid and PRPP (see, e.g., FIGS. 13A and 13B), confirming the substrate specificity of this enzyme. In isolated reactions, it was also confirmed that Nmnat catalyzed the synthesis of NAD from NMN and ATP.
Name
Tris-HCl
Quantity
0 (± 1) mol
Type
reactant
Reaction Step One





Name

Name
Identifiers


|
REACTION_CXSMILES
|
C(O)C(N)(CO)CO.Cl.[Mg+2].[Cl-].[Cl-].[C:13]([NH2:21])(=[O:20])[C:14]1[CH:19]=[CH:18][CH:17]=[N:16][CH:15]=1.[CH2:22]([O:39][P:40]([OH:43])([OH:42])=[O:41])[C@H:23]1[O:27][C@H:26]([O:28][P:29]([O:32][P:33]([OH:36])([OH:35])=[O:34])([OH:31])=[O:30])[C@H:25]([OH:37])[C@@H:24]1[OH:38]>>[CH:18]1[CH:17]=[N+:16]([CH:26]2[O:27][C@H:23]([CH2:22][O:39][P:40]([O-:43])([OH:42])=[O:41])[C@@H:24]([OH:38])[C@H:25]2[OH:37])[CH:15]=[C:14]([C:13]([NH2:21])=[O:20])[CH:19]=1.[C:13]([NH2:21])(=[O:20])[C:14]1[CH:19]=[CH:18][CH:17]=[N:16][CH:15]=1.[CH2:22]([O:39][P:40]([OH:43])([OH:42])=[O:41])[C@H:23]1[O:27][C@H:26]([O:28][P:29]([O:32][P:33]([OH:36])([OH:35])=[O:34])([OH:31])=[O:30])[C@H:25]([OH:37])[C@@H:24]1[OH:38] |f:0.1,2.3.4|
|
Inputs


Step One
|
Name
|
Tris-HCl
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C(CO)(CO)N)O.Cl
|
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
[Mg+2].[Cl-].[Cl-]
|
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)N
|
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C([C@@H]1[C@H]([C@H]([C@H](O1)OP(=O)(O)OP(=O)(O)O)O)O)OP(=O)(O)O
|
Conditions


Other
|
Conditions are dynamic
|
1
|
|
Details
|
See reaction.notes.procedure_details.
|
Workups


CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
Nampt reaction products
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
The Nampt reaction
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
The reaction was terminated
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
by adding 125 μl of 1 M HClO4
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
Protein was then precipitated at 18,000 g, and 500 μl of the supernatant
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
After centrifugation, 100 μl of sample was mixed with 400 μl of Buffer A (50 mM K2PO4/KHPO4, pH 7.0)
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
The products from Nampt reaction
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
Results of HPLC detection of Nampt reaction products
|
Outcomes


Product
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
C1=CC(=C[N+](=C1)C2[C@@H]([C@@H]([C@H](O2)COP(=O)(O)[O-])O)O)C(=O)N
|
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)N
|
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
C([C@@H]1[C@H]([C@H]([C@H](O1)OP(=O)(O)OP(=O)(O)O)O)O)OP(=O)(O)O
|
Source


|
Source
|
Open Reaction Database (ORD) |
|
Description
|
The Open Reaction Database (ORD) is an open-access schema and infrastructure for structuring and sharing organic reaction data, including a centralized data repository. The ORD schema supports conventional and emerging technologies, from benchtop reactions to automated high-throughput experiments and flow chemistry. Our vision is that a consistent data representation and infrastructure to support data sharing will enable downstream applications that will greatly improve the state of the art with respect to computer-aided synthesis planning, reaction prediction, and other predictive chemistry tasks. |
