Pralatrexate
概要
説明
Pralatrexate is a novel antifolate compound used primarily for the treatment of relapsed or refractory peripheral T-cell lymphoma. It was approved by the U.S. Food and Drug Administration in 2009. This compound is designed to have a high affinity for the reduced folate carrier, which allows it to be efficiently internalized by tumor cells .
作用機序
Target of Action
Pralatrexate is an antifolate, a class of drugs that inhibit folate metabolism, which is essential for many cellular processes, including cellular proliferation . The primary targets of this compound are the dihydrofolate reductase (DHFR) and the reduced folate carrier type 1 (RFC-1) . DHFR is an enzyme involved in the synthesis of nucleic acid precursors and several amino acids, making it critical for the de novo synthesis of DNA and proliferation of mammalian cells . RFC-1 is a protein that is overexpressed in malignant cells and is upregulated by oncogenes . It efficiently internalizes natural folates and antifolates, including this compound .
Mode of Action
This compound competitively inhibits DHFR, thereby impeding the synthesis of amino acids and nucleic acid . It is also a competitive inhibitor for polyglutamylation by folylpolyglutamate synthase (FPGS), which increases cellular retention of this compound for extended drug action and impedes the uptake of folate, also a substrate of FPGS, to further inhibit folate metabolism in cancer cells . This compound is designed to have a higher affinity for RFC-1, leading to better accumulation in cancer cells compared to other antifolates .
Biochemical Pathways
This compound inhibits folate-mediated one-carbon metabolism, a biochemical pathway critical for the synthesis of nucleic acid precursors and several amino acids . This inhibition disrupts the de novo synthesis of DNA, leading to the interruption of RNA synthesis, DNA replication, and ultimately, apoptosis .
Pharmacokinetics
This compound is actively transported across the cellular membrane through RFC-1 . Its retention in the cytoplasm depends upon polyglutamylation of the antifolate compound, which is catalyzed by FPGS . This reaction both increases cellular retention of this compound for extended drug action and impedes the uptake of folate . The rate of this compound influx is nearly 14-fold more than that of methotrexate .
Result of Action
The molecular effect of this compound’s action is the interruption of RNA synthesis and DNA replication, leading to apoptosis . On a cellular level, this compound induces concentration-dependent apoptotic cell death . It has been shown to be very active across many lymphoid malignancies, including chemotherapy-resistant T-cell lymphoma .
Action Environment
The efficacy and stability of this compound can be influenced by environmental factors such as the presence of other drugs. For example, coadministration of this compound with probenecid increased this compound plasma concentrations . Additionally, the resistance mechanisms of this compound were associated with reduced cellular uptake of this compound and/or overexpression of DNA-methyltransferase 3β (DNMT3B) . Epigenetic alterations were also considered to play a role in the resistance mechanism .
生化学分析
Biochemical Properties
Pralatrexate inhibits folate-mediated one-carbon metabolism . It is actively transported across the cellular membrane through the RFC, a member of the solute carrier transmembrane protein family . Retention in the cytoplasm depends upon polyglutamylation of the antifolate compound, which is catalyzed by folylpolyglutamate synthetase (FPGS) .
Cellular Effects
This compound has demonstrated varying degrees of efficacy in peripheral T-cell lymphoma, with response rates differing between the multiple subtypes of the disease . In PDX-resistant T-cell acute lymphoblastic leukemia cell lines, the combination of decitabine (DAC) and this compound exhibited a potent synergistic effect .
Molecular Mechanism
This compound is a folate analog metabolic inhibitor that competitively inhibits dihydrofolate reductase . It also competes for enzymatic processing by FPGS with folate to increase cellular retention .
Temporal Effects in Laboratory Settings
The best therapeutic effects were obtained with the sequence of this compound → gemcitabine . Complete remissions were only appreciated in animals receiving this compound followed by gemcitabine .
Dosage Effects in Animal Models
Preliminary studies of this compound in animal models highlighted that, at high concentrations, this compound induced mucosal inflammation and destruction of the gastrointestinal epithelium . Multiple doses of the drug led to reversible anemia, neutropenia, and leukopenia in dogs .
Metabolic Pathways
This compound is involved in the folate metabolic pathway . It competes with folate for enzymatic processing by FPGS, leading to increased cellular retention of this compound .
Transport and Distribution
This compound is actively transported across the cellular membrane through the RFC . Its retention in the cytoplasm depends upon polyglutamylation of the antifolate compound, which is catalyzed by FPGS .
Subcellular Localization
This compound is localized within the cytoplasm of the cell . Its retention in the cytoplasm depends upon polyglutamylation of the antifolate compound, which is catalyzed by FPGS .
準備方法
Synthetic Routes and Reaction Conditions: The synthesis of Pralatrexate involves several key steps. One method starts with 10-propargyl-10-methoxycarbonyl-4-deoxy-4-amino-10-deaza pteroic acid methyl ester as the starting material. This compound undergoes a saponification reaction to yield 4-(2-carboxy-1-(2,4-diaminopteridine-6-yl)pent-4-yn-2-yl)benzoic acid. This intermediate is then decarboxylated to produce 4-(1-(2,4-diaminopteridine-6-yl)pent-4-yn-2-yl)benzoic acid, which reacts with L-diethyl glutamate to form 10-propargyl-10-deaza aminopterin diethyl ester. Finally, a saponification reaction is performed to obtain this compound .
Industrial Production Methods: The industrial production of this compound follows similar synthetic routes but is optimized for higher yields and purity. The process involves the use of polar solvents like dehydrated alcohol and basic solutions such as sodium hydroxide to facilitate the reactions .
化学反応の分析
Types of Reactions: Pralatrexate undergoes various chemical reactions, including:
Oxidation: this compound can be oxidized under specific conditions to form different derivatives.
Reduction: Reduction reactions can modify the pteridine ring system.
Substitution: Substitution reactions can occur at the amino groups or the pteridine ring.
Common Reagents and Conditions:
Oxidation: Common oxidizing agents include hydrogen peroxide and potassium permanganate.
Reduction: Reducing agents like sodium borohydride are used.
Substitution: Reagents such as alkyl halides and acyl chlorides are employed.
Major Products: The major products formed from these reactions include various derivatives of this compound that can have different pharmacological properties .
科学的研究の応用
Pralatrexate has a wide range of scientific research applications:
Chemistry: Used as a model compound to study antifolate mechanisms and develop new antifolate drugs.
Biology: Helps in understanding folate metabolism and transport in cells.
Medicine: Primarily used in the treatment of peripheral T-cell lymphoma. .
Industry: this compound’s synthesis and production methods are studied to improve industrial processes and reduce costs
類似化合物との比較
Methotrexate: An older antifolate used in various cancers and autoimmune diseases.
Pemetrexed: Another antifolate used in the treatment of non-small-cell lung cancer and mesothelioma.
Raltitrexed: Used in the treatment of colorectal cancer.
Lometrexol: An investigational antifolate with potential anticancer properties.
Uniqueness of Pralatrexate: this compound is unique due to its high affinity for the reduced folate carrier, which allows for efficient internalization and retention in tumor cells. This property makes it more effective in targeting cancer cells compared to other antifolates .
特性
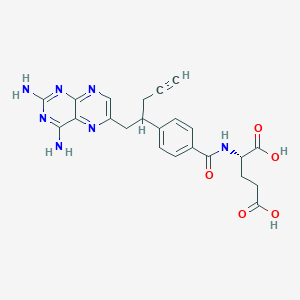
IUPAC Name |
(2S)-2-[[4-[1-(2,4-diaminopteridin-6-yl)pent-4-yn-2-yl]benzoyl]amino]pentanedioic acid | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C23H23N7O5/c1-2-3-14(10-15-11-26-20-18(27-15)19(24)29-23(25)30-20)12-4-6-13(7-5-12)21(33)28-16(22(34)35)8-9-17(31)32/h1,4-7,11,14,16H,3,8-10H2,(H,28,33)(H,31,32)(H,34,35)(H4,24,25,26,29,30)/t14?,16-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
OGSBUKJUDHAQEA-WMCAAGNKSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C#CCC(CC1=CN=C2C(=N1)C(=NC(=N2)N)N)C3=CC=C(C=C3)C(=O)NC(CCC(=O)O)C(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C#CCC(CC1=CN=C2C(=N1)C(=NC(=N2)N)N)C3=CC=C(C=C3)C(=O)N[C@@H](CCC(=O)O)C(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C23H23N7O5 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID3048578 | |
| Record name | Pralatrexate | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3048578 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
477.5 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
Practically insoluble in shloroform and ethanol, Soluble in aqueous solutions at pH 6.5 or higher | |
| Record name | Pralatrexate | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7786 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Mechanism of Action |
Pralatrexate is a folate analog metabolic inhibitor that competitively inhibits dihydrofolate reductase (DHFR) selectively in cancer cells overexpressing the reduced folate carrier protein-1 (RFC-1). Folate is a water-soluble vitamin required for DNA synthesis and maintenance as well as DNA, RNA, and protein methylation. As cancer cells are rapidly replicating, they require a lot of folates to accommodate an accelerated cell division and DNA and protein modification for cellular transformation. Therefore, interruption with folate metabolism can inhibit tumor growth. Additionally, pralatrexate also undergoes polyglutamylation catalyzed by folyopolyglutamate synthase (FPGS). This reaction both increases cellular retention of pralatrexate for extended drug action and impedes the uptake of folate, also a substrate of FPGS, to further inhibit folate metabolism in cancer cells., Pralatrexate is a folate analogue metabolic inhibitor that competitively inhibits dihydrofolate reductase. It is also a competitive inhibitor for polyglutamylation by the enzyme folylpolyglutamyl synthetase. This inhibition results in the depletion of thymidine and other biological molecules the synthesis of which depends on single carbon transfer., This study evaluated mechanistic differences of pralatrexate, methotrexate, and pemetrexed. Inhibition of dihydrofolate reductase (DHFR) was quantified using recombinant human DHFR. Cellular uptake and folylpolyglutamate synthetase (FPGS) activity were determined using radiolabeled pralatrexate, methotrexate, and pemetrexed in NCI-H460 non-small cell lung cancer (NSCLC) cells. The tumor growth inhibition (TGI) was assessed using MV522 and NCI-H460 human NSCLC xenografts. Apparent K ( i ) values for DHFR inhibition were 45, 26, and >200 nM for pralatrexate, methotrexate, and pemetrexed, respectively. A significantly greater percentage of radiolabeled pralatrexate entered the cells and was polyglutamylatated relative to methotrexate or pemetrexed. In vivo, pralatrexate showed superior anti-tumor activity in both NSCLC models, with more effective dose-dependent TGI in the more rapidly growing NCI-H460 xenografts. Pralatrexate demonstrated a distinct mechanistic and anti-tumor activity profile relative to methotrexate and pemetrexed. Pralatrexate exhibited enhanced cellular uptake and increased polyglutamylation, which correlated with increased TGI in NSCLC xenograft models. | |
| Record name | Pralatrexate | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB06813 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Pralatrexate | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7786 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Off-white to yellow solid | |
CAS No. |
146464-95-1 | |
| Record name | Pralatrexate | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=146464-95-1 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Pralatrexate [USAN:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0146464951 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Pralatrexate | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB06813 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Pralatrexate | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=754230 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Pralatrexate | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3048578 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | (2S)-2-[[4-[4-[(1RS)-1-[(2,4-diaminopteridin-6-yl)methyl]but-3-ynyl]benzoyl]amino]pentanedioc acid | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | PRALATREXATE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/A8Q8I19Q20 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Pralatrexate | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7786 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。