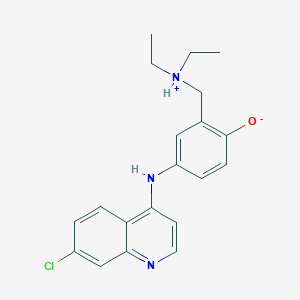
アモジアキン
概要
説明
アモジアキンは、4-アミノキノリン系に属する合成化合物です。 主に抗マラリア薬として使用され、特にクロロキンなどの他の抗マラリア薬に対する耐性が発達した地域では、マラリア原虫ファルシパルムに効果があります 。 アモジアキンは、その有効性を高め、耐性のリスクを減らすために、アルテスネートとの併用療法でよく使用されます 。
作用機序
アモジアキンは、マラリア原虫のヘムポリメラーゼ活性を阻害することで、抗マラリア効果を発揮します。この阻害により、遊離ヘムが蓄積し、原虫にとって毒性となります。 薬物は遊離ヘムに結合し、原虫がそれを毒性の低い形に変換するのを阻害し、膜機能を破壊し、原虫の死滅につながります 。 主要な分子標的はFe(II)-プロトポルフィリンIXです 。
科学的研究の応用
Amodiaquine has a wide range of scientific research applications:
生化学分析
Biochemical Properties
Amodiaquine interacts with various biomolecules in its role as an antimalarial agent. The mechanism of plasmodicidal action of amodiaquine is not completely certain. Like other quinoline derivatives, it is thought to inhibit heme polymerase activity . This results in the accumulation of free heme, which is toxic to the parasites. Amodiaquine binds the free heme, preventing the parasite from converting it to a form less toxic . This drug-heme complex is toxic and disrupts membrane function .
Cellular Effects
Amodiaquine has significant effects on various types of cells and cellular processes. It is known to depress cardiac muscle, impair cardiac conductivity, and produce vasodilatation with resultant hypotension . It also depresses respiration and can cause diplopia, dizziness, and nausea .
Molecular Mechanism
The molecular mechanism of action of amodiaquine involves its interaction with free heme. Amodiaquine is thought to inhibit heme polymerase activity, leading to the accumulation of free heme . The drug then binds the free heme, preventing the parasite from converting it to a less toxic form . This drug-heme complex is toxic and disrupts membrane function .
Temporal Effects in Laboratory Settings
Amodiaquine has shown consistent effects over time in laboratory settings. A study of the pharmacokinetic properties of amodiaquine provided evidence of high cure rates with exposure to the drug being remarkably consistent across all age groups .
Dosage Effects in Animal Models
While specific studies on the dosage effects of amodiaquine in animal models are limited, it is known that the cardiovascular effects of amodiaquine have been recognized from the earliest studies in animal models .
Metabolic Pathways
Amodiaquine is bioactivated hepatically to its primary metabolite, N-desethylamodiaquine, by the cytochrome p450 enzyme CYP2C8 . This metabolite is largely responsible for the antimalarial effect of the drug .
Transport and Distribution
Amodiaquine is likely to be widely distributed into body tissues, particularly in the liver, spleen, kidney, lungs, brain, and spinal cord .
Subcellular Localization
The subcellular localization of amodiaquine is not well characterized. Given its mechanism of action, it is likely that amodiaquine and its active metabolite are localized in the cytoplasm where they can interact with free heme .
準備方法
合成経路と反応条件
アモジアキンは、4,7-ジクロロキノリンと4-アミノフェノールを塩基の存在下で反応させる多段階プロセスにより合成されます。 反応は求核置換反応を経て進行し、中間体が生成され、その後ジエチルアミンとさらに反応させるとアモジアキンが生成されます 。
工業的製造方法
アモジアキンの工業的製造では、実験室での合成と同じ反応条件を用いた大規模合成が行われます。 このプロセスは、高収率と高純度のために最適化されており、最終製品が医薬品基準を満たすように、厳しい品質管理が行われています 。
化学反応の分析
反応の種類
アモジアキンは、次のようなさまざまな化学反応を起こします。
酸化: アモジアキンは酸化されて、主要な代謝産物であるN-デスエチルアモジアキンを形成することができます.
還元: 還元反応はあまり一般的ではありませんが、特定の条件下で起こることがあります。
一般的な試薬と条件
酸化: 一般的な酸化剤には、過酸化水素や過マンガン酸カリウムなどがあります。
還元: 水素化ホウ素ナトリウムなどの還元剤を使用することができます。
生成される主な生成物
アモジアキンの酸化から生成される主な生成物は、N-デスエチルアモジアキンであり、抗マラリア活性を持っています 。
科学研究への応用
アモジアキンは、さまざまな科学研究に広く応用されています。
類似化合物との比較
アモジアキンは、クロロキン、メフロキン、ピペラキンなどの他の4-アミノキノリン化合物と似ています 。 クロロキン耐性マラリア原虫ファルシパルムに対して効果があるという独自の特性を持っています 。 クロロキンとは異なり、アモジアキンは、その有効性を高め、耐性を減らすために、併用療法でよく使用されます 。
類似化合物のリスト
- クロロキン
- メフロキン
- ピペラキン
- ルメファントリン
- プリマキン
- タフェノキン
特性
IUPAC Name |
4-[(7-chloroquinolin-4-yl)amino]-2-(diethylaminomethyl)phenol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-11-16(6-8-20(14)25)23-18-9-10-22-19-12-15(21)5-7-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
OVCDSSHSILBFBN-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCN(CC)CC1=C(C=CC(=C1)NC2=C3C=CC(=CC3=NC=C2)Cl)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C20H22ClN3O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID2022597 | |
| Record name | Amodiaquine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2022597 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
355.9 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Amodiaquine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014751 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
24.9 [ug/mL] (The mean of the results at pH 7.4), 8.80e-03 g/L | |
| Record name | SID50085969 | |
| Source | Burnham Center for Chemical Genomics | |
| URL | https://pubchem.ncbi.nlm.nih.gov/bioassay/1996#section=Data-Table | |
| Description | Aqueous solubility in buffer at pH 7.4 | |
| Record name | Amodiaquine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014751 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
The mechanism of plasmodicidal action of amodiaquine is not completely certain. Like other quinoline derivatives, it is thought to inhibit heme polymerase activity. This results in accumulation of free heme, which is toxic to the parasites. The drug binds the free heme preventing the parasite from converting it to a form less toxic. This drug-heme complex is toxic and disrupts membrane function., Amodiaquine is a Mannich base 4-aminoquinoline with a mode of action similar to that of chloroquine. It is effective against some chloroquine-resistant strains of P. falciparum, although there is cross-resistance., The 4-aminoquinoline derivatives appear to bind to nucleoproteins and interfere with protein synthesis in susceptible organisms; the drugs intercalate readily into double-stranded DNA and inhibit both DNA and RNA polymerase. In addition, the drugs apparently concentrate in parasite digestive vacuoles, increase the pH of the vacuoles, and interfere with the parasite's ability to metabolize and utilize erythrocyte hemoglobin. Plasmodial forms that do not have digestive vacuoles and do not utilize hemoglobin, such as exoerythrocytic forms, are not affected by /these medications/., The 4-aminoquinoline derivatives ... have anti-inflammatory activity; however, the mechanism(s) of action of the drugs in the treatment of rheumatoid arthritis and lupus erythematosus has not been determined. /4-aminoquinoline derivatives/ reportedly antagonizes histamine in vitro, has antiserotonin effects, and inhibits prostaglandin effects in mammalian cells presumably by inhibiting conversion of arachidonic acid to prostaglandin F2., The mode of action of amodiaquine has not yet been determined. 4-Aminoquinolines depress cardiac muscle, impair cardiac conductivity, and produce vasodilatation with resultant hypotension; they depress respiration and cause diplopia, dizziness and nausea. | |
| Record name | Amodiaquine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00613 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | AMODIAQUINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7457 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Crystals from absolute ethanol | |
CAS No. |
86-42-0 | |
| Record name | Amodiaquine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=86-42-0 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Amodiaquine [USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000086420 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Amodiaquine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00613 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | amodiaquine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=13453 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Amodiaquine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2022597 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Amodiaquine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.001.518 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | AMODIAQUINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/220236ED28 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | AMODIAQUINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7457 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Amodiaquine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014751 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
206-208, 208 °C (decomposes), Yellow crystals from methanol. Melting point 243 °C. Slightly soluble in water and alcohol /Amodiaquine dihydrochloride hemihydrate/, 208 °C | |
| Record name | Amodiaquine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00613 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | AMODIAQUINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7457 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Amodiaquine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014751 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。


![5-[(6-Aminopurin-9-yl)methyl]-5-methyl-3-methylideneoxolan-2-one](/img/structure/B18304.png)