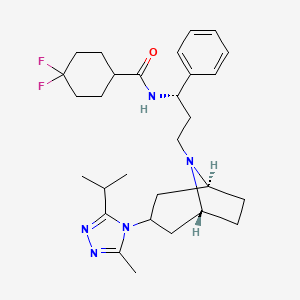
Maraviroc
説明
Maraviroc is a chemokine receptor antagonist developed by Pfizer. It is used in combination with other antiretroviral medications to treat CCR5-tropic human immunodeficiency virus type 1 (HIV-1) infection. This compound works by interfering with the interaction between HIV and the CCR5 receptor, thereby preventing the virus from entering human cells .
準備方法
The synthesis of Maraviroc involves several steps. One method includes the “borrowing hydrogen” or “hydrogen autotransfer” method. This process involves the reaction of the tropane triazole with the (S)-amido alcohol, catalyzed by a suitable catalyst . Another method involves the preparation of a tropane derivative by reacting specific compounds under controlled conditions . Industrial production methods focus on optimizing the yield and purity of the final product through improved isolation and purification techniques .
化学反応の分析
Maraviroc undergoes various chemical reactions, including substitution and reduction reactions. Common reagents used in these reactions include hydrogen gas and suitable catalysts. The major products formed from these reactions are derivatives of the original compound, which may have different pharmacological properties .
科学的研究の応用
Maraviroc has a wide range of scientific research applications. In medicine, it is primarily used as an antiretroviral agent to manage HIV-1 infection. It has shown efficacy in reducing viral load and improving immune function in patients . In addition to its use in HIV treatment, this compound has been studied for its potential anti-inflammatory effects and its ability to alleviate sepsis-related organ damage . In chemistry and biology, this compound serves as a valuable tool for studying the CCR5 receptor and its role in various physiological processes .
作用機序
Maraviroc is an entry inhibitor that works by blocking HIV from entering human cells. Specifically, it is a selective, slowly reversible antagonist of the interaction between the human CCR5 receptor and the HIV-1 gp120 protein . By binding to the CCR5 receptor, this compound prevents the virus from attaching to and entering the host cell, thereby inhibiting viral replication .
類似化合物との比較
Maraviroc is unique among antiretroviral agents because it targets a host protein, the CCR5 receptor, rather than a viral protein. Similar compounds include other CCR5 antagonists and entry inhibitors. For example, diazabicyclo analogues of this compound have been synthesized and tested for their antiviral activity . These analogues show varying degrees of efficacy in inhibiting HIV-1 entry into host cells. This compound’s specificity for the CCR5 receptor and its ability to block HIV entry make it a valuable addition to the arsenal of antiretroviral drugs .
特性
IUPAC Name |
4,4-difluoro-N-[(1S)-3-[(1S,5R)-3-(3-methyl-5-propan-2-yl-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octan-8-yl]-1-phenylpropyl]cyclohexane-1-carboxamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C29H41F2N5O/c1-19(2)27-34-33-20(3)36(27)25-17-23-9-10-24(18-25)35(23)16-13-26(21-7-5-4-6-8-21)32-28(37)22-11-14-29(30,31)15-12-22/h4-8,19,22-26H,9-18H2,1-3H3,(H,32,37)/t23-,24+,25?,26-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
GSNHKUDZZFZSJB-HLMSNRGBSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1=NN=C(N1C2CC3CCC(C2)N3CCC(C4=CC=CC=C4)NC(=O)C5CCC(CC5)(F)F)C(C)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC1=NN=C(N1C2C[C@H]3CC[C@@H](C2)N3CC[C@@H](C4=CC=CC=C4)NC(=O)C5CCC(CC5)(F)F)C(C)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C29H41F2N5O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID8048949 | |
| Record name | Maraviroc | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8048949 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
513.7 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Maraviroc | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015584 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
1.06e-02 g/L | |
| Record name | Maraviroc | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015584 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Maraviroc is an entry inhibitor and works by blocking HIV from entering human cells. Specifically maraviroc is a selective, slowly reversible, small molecule antagonist of the interaction between human CCR5 and HIV-1 gp120. Maraviroc selectively binds to the human chemokine receptor CCR5 present on the membrane of CD4 cells (T-cells), preventing the interaction of HIV-1 gp120 and CCR5 necessary for CCR5-tropic HIV-1 to enter cells., Maraviroc, a synthetic antiretroviral agent, is an HIV entry inhibitor. The drug is a small molecule CCR5 antagonist. HIV enters host cells by attaching to the CD4+ T-cell receptor using 1 of 2 chemokine co-receptors, CCR5 or CXCR4. Maraviroc selectively binds to CCR5 on the cell membrane and prevents the interaction of HIV-1 glycoprotein 120 and CCR5 necessary for CCR5-tropic HIV-1 to enter cells. Maraviroc does not inhibit entry of CXCR4-tropic and dual/mixed-tropic HIV-1 into cells. CCR5 is a co-receptor for the most commonly transmitted HIV-1 strains that predominate during the early stages of infection; this form remains the dominant form in many patients with late-stage infection. Maraviroc is active against some strains of HIV-1 resistant to nucleoside reverse transcriptase inhibitors (NRTIs), nonnucleoside reverse transcriptase inhibitors (NNRTIs), HIV protease inhibitors (PIs), and HIV entry and fusion inhibitors (enfuvirtide). HIV-1 strains with reduced susceptibility to maraviroc have been produced in vitro and have emerged during maraviroc therapy., Maraviroc is a member of a therapeutic class called CCR5 co-receptor antagonists. Maraviroc selectively binds to the human chemokine receptor CCR5 present on the cell membrane, preventing the interaction of HIV-1 gp120 and CCR5 necessary for CCR5-tropic HIV-1 to enter cells. CXCR4-tropic and dual-tropic HIV-1 entry is not inhibited by maraviroc., Maraviroc inhibits the replication of CCR5-tropic laboratory strains and primary isolates of HIV-1 in models of acute peripheral blood leukocyte infection. The mean EC50 value (50% effective concentration) for maraviroc against HIV-1 group M isolates (subtypes A to J and circulating recombinant form AE) and group O isolates ranged from 0.1 to 4.5 nM (0.05 to 2.3 ng/mL) in cell culture. ... Maraviroc was not active against CXCR4-tropic and dual-tropic viruses (EC50 value >10 uM). The antiviral activity of maraviroc against HIV-2 has not been evaluated., Maraviroc (MVC, Celsentri) is an allosteric and reversible inhibitor of the CCR5 chemokine coreceptor. ... MVC exclusively inhibits the replication of R5- tropic HIV-1 variants after binding to the transmembrane CCR5 receptor cavity., For more Mechanism of Action (Complete) data for Maraviroc (6 total), please visit the HSDB record page. | |
| Record name | Maraviroc | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB04835 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Maraviroc | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8021 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
White solid from toluene/hexane (2:1), White- to pale-colored powder | |
CAS No. |
376348-65-1, 2414315-81-2 | |
| Record name | Maraviroc [INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0376348651 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Maraviroc | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB04835 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Maraviroc | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8048949 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Maraviroc | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/MD6P741W8A | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Maraviroc, endo- | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/A3C7SRV8DV | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Maraviroc | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8021 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Maraviroc | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015584 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
197-198 °C | |
| Record name | Maraviroc | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8021 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。




![10-(4-aminobutyl)-N-(1-amino-3-hydroxy-1-oxobutan-2-yl)-19-[(2-amino-3-naphthalen-2-ylpropanoyl)amino]-16-[(4-hydroxyphenyl)methyl]-13-(1H-indol-3-ylmethyl)-6,9,12,15,18-pentaoxo-7-propan-2-yl-1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carboxamide](/img/structure/B10761210.png)