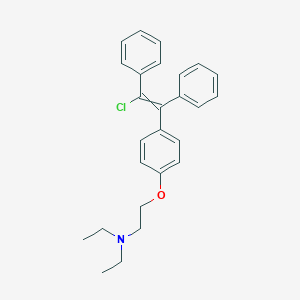
Clomifene
概要
説明
It belongs to the selective estrogen receptor modulator (SERM) family and functions by stimulating the release of gonadotropins, which in turn induce ovulation . Clomifene is taken orally and has been a cornerstone in assisted reproductive technology since its approval in the United States in 1967 .
作用機序
Target of Action
Clomiphene primarily targets estrogen-receptor-containing tissues, including the hypothalamus, pituitary, ovary, endometrium, vagina, and cervix . It competes with estrogen for estrogen-receptor-binding sites and may delay replenishment of intracellular estrogen receptors .
Mode of Action
Clomiphene acts as a selective estrogen receptor modulator (SERM). It can lead to multiple ovulation, hence increasing the risk of conceiving twins . The first endocrine event in response to a course of clomiphene therapy is an increase in the release of pituitary gonadotropins. This initiates steroidogenesis and folliculogenesis, resulting in the growth of the ovarian follicle and an increase in the circulating level of estradiol .
Pharmacokinetics
Clomiphene is readily absorbed and undergoes extensive metabolism in the liver via the CYP2D6 pathway . It has a high bioavailability (>90%) and a long elimination half-life of 4-7 days . It is primarily excreted in feces (42%), with some excretion in urine (8%) .
Result of Action
It can lead to multiple ovulation, hence increasing the risk of conceiving twins . There may be an increased risk of ovarian cancer and weight gain .
Action Environment
The action of clomiphene can be influenced by various environmental factors. . This is because these conditions can affect the metabolism and efficacy of clomiphene. Furthermore, the drug’s action can be influenced by the patient’s hormonal environment, which can vary based on factors such as age, overall health, and presence of certain medical conditions .
生化学分析
Biochemical Properties
Clomiphene interacts with various enzymes, proteins, and other biomolecules. It is biotransformed to 22 metabolites, with phase I reactions being catalyzed mainly by CYP3A4 and CYP2D6 isoforms and, to a lesser degree, by CYP3A5, CYP2B6, CYP2C9, CYP2C19 isoforms .
Cellular Effects
Clomiphene has significant effects on various types of cells and cellular processes. It influences cell function, including impacts on cell signaling pathways, gene expression, and cellular metabolism .
Molecular Mechanism
Clomiphene exerts its effects at the molecular level through binding interactions with biomolecules, enzyme inhibition or activation, and changes in gene expression .
Temporal Effects in Laboratory Settings
The effects of Clomiphene change over time in laboratory settings. Information on the product’s stability, degradation, and any long-term effects on cellular function observed in in vitro or in vivo studies is still being researched .
Dosage Effects in Animal Models
The effects of Clomiphene vary with different dosages in animal models. This includes any threshold effects observed in these studies, as well as any toxic or adverse effects at high doses .
Metabolic Pathways
Clomiphene is involved in various metabolic pathways, interacting with enzymes or cofactors. It also affects metabolic flux or metabolite levels .
Transport and Distribution
Clomiphene is transported and distributed within cells and tissues. It interacts with transporters or binding proteins, affecting its localization or accumulation .
Subcellular Localization
This could include any targeting signals or post-translational modifications that direct it to specific compartments or organelles .
準備方法
Synthetic Routes and Reaction Conditions: The synthesis of clomifene involves the reaction of 2-chloro-1,2-diphenylethylene with 4-(2-chloro-1,2-diphenylethenyl)phenol in the presence of a base, followed by the reaction with diethylamine . The process can be optimized using acetic acid or trifluoroacetic acid as solvents .
Industrial Production Methods: Industrial production of this compound typically involves the use of acetic acid or trifluoroacetic acid in specific volumes to ensure high yield and purity . The process is designed to produce both the trans and cis isomers of this compound, with trans-clomifene being the more active form .
化学反応の分析
Types of Reactions: Clomifene undergoes several types of chemical reactions, including:
Oxidation: this compound can be oxidized to form 4-hydroxythis compound and 4-hydroxy-N-desethylthis compound.
Reduction: Reduction reactions are less common but can occur under specific conditions.
Substitution: this compound can undergo substitution reactions, particularly involving the phenoxy group.
Common Reagents and Conditions:
Oxidation: Common oxidizing agents include hydrogen peroxide and potassium permanganate.
Substitution: Reagents such as sodium hydroxide and potassium carbonate are often used.
Major Products:
Oxidation Products: 4-hydroxythis compound and 4-hydroxy-N-desethylthis compound.
Substitution Products: Various substituted phenoxy derivatives.
科学的研究の応用
Clomifene has a wide range of scientific research applications:
Chemistry: Used as a model compound to study the behavior of selective estrogen receptor modulators.
Biology: Investigated for its effects on estrogen receptors in various tissues.
Medicine: Primarily used to induce ovulation in women with infertility issues.
Industry: Employed in the development of fertility treatments and hormone therapies.
類似化合物との比較
Tamoxifen: Another selective estrogen receptor modulator used primarily in the treatment of breast cancer.
Toremifene: Similar to tamoxifen, used in the treatment of metastatic breast cancer.
Enclomiphene: A purified isomer of clomifene, used in testosterone replacement therapy.
Comparison:
Tamoxifen vs. This compound: Both are SERMs, but tamoxifen is more commonly used in cancer treatment, while this compound is used in fertility treatments.
Toremifene vs. This compound: Toremifene has a similar mechanism but is used for different clinical indications.
Enclomiphene vs. This compound: Enclomiphene is a more targeted form of this compound, with fewer side effects related to mood.
This compound’s unique ability to induce ovulation and increase testosterone levels makes it a versatile compound in both reproductive medicine and hormone therapy.
特性
IUPAC Name |
2-[4-[(Z)-2-chloro-1,2-diphenylethenyl]phenoxy]-N,N-diethylethanamine | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C26H28ClNO/c1-3-28(4-2)19-20-29-24-17-15-22(16-18-24)25(21-11-7-5-8-12-21)26(27)23-13-9-6-10-14-23/h5-18H,3-4,19-20H2,1-2H3/b26-25- | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
GKIRPKYJQBWNGO-QPLCGJKRSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCN(CC)CCOC1=CC=C(C=C1)C(=C(C2=CC=CC=C2)Cl)C3=CC=CC=C3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CCN(CC)CCOC1=CC=C(C=C1)/C(=C(/C2=CC=CC=C2)\Cl)/C3=CC=CC=C3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C26H28ClNO | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID601317947 | |
| Record name | Zuclomiphene | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID601317947 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
406.0 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
SLIGHTLY SOL IN WATER (1 IN 900), ETHANOL (1 IN 40) AND CHLOROFORM (1 IN 800); FREELY SOL IN METHANOL; PRACTICALLY INSOL IN DIETHYL ETHER /CITRATE/ | |
| Record name | CLOMIPHENE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3039 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Mechanism of Action |
CLOMIPHENE...AFFECTS SPERMIOGENESIS @ PRIMARY SPERMATOCYTE LEVEL. DOSE USED WAS 7.25 MG/DAY. EFFECT IS THOUGHT TO BE DUE TO ESTROGENICITY MEDIATED VIA HYPOTHALAMO-HYPOPHYSEAL AXIS..., The antiestrogens tamoxifen and clomiphene are used primarily for the treatment of breast cancer and female infertility, respectively. These agents are used therapeutically for their antiestrogenic actions, but they can produce estrogenic as well as antiestrogenic effects. Both agents competitively block estradiol binding to its receptor, but the specific pharmacological activity they produce depends upon the species, the tissue, and the cellular endpoint measured. Consequently, these agents act as antagonists, agonists, or partial agonists depending upon the context in which they are used., Clomiphene and tamoxifen clearly bind to the estrogen receptor and can prevent the binding of estrogens. However, there are indications that the drugs and estradiol may interact with overlapping but slightly different regions of the ligand binding site of the estrogen receptor. Depending upon the specific cellular context and gene in question, antiestrogen binding may yield a receptor complex that has full, partial, or no intrinsic activity., Clomiphene may stimulate ovulation in women with an intact hypothalamic-pituitary-ovarian axis and adequate endogenous estrogens who have failed to ovulate. In these cases, it is thought that the drug opposes the negative feedback of endogenous estrogens resulting in increased gonadotropin secretion and ovulation. Most studies indicate that clomiphene increases the amplitude of LH and FSH pulses, without a change in pulse frequency. This suggests the drug is acting largely at the pituitary level to block inhibitory actions of estrogen on gonadotropin release from the gland and/or is somehow causing the hypothalamus to release larger amounts of gonadotropin-releasing hormone per pulse., Initial animal studies with clomiphene showed slight estrogenic activity and moderate antiestrogenic activity, but the most striking effect was the inhibition of the pituitary's gonadotropic function. In both male and female animals, the compound acted as a contraceptive. | |
| Record name | CLOMIPHENE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3039 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
CAS No. |
15690-55-8, 911-45-5 | |
| Record name | Zuclomiphene | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=15690-55-8 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Zuclomiphene [USAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0015690558 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Zuclomiphene | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID601317947 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Clomifene | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.011.826 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | ZUCLOMIPHENE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/3JU1DU3652 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | CLOMIPHENE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3039 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Melting Point |
MP: 116.5-118 °C /CITRATE/ | |
| Record name | CLOMIPHENE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3039 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Synthesis routes and methods
Procedure details




Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: How does clomifene citrate interact with its target in the body?
A1: this compound citrate acts as a selective estrogen receptor modulator (SERM). [] It binds to estrogen receptors in the hypothalamus, blocking the negative feedback loop of estrogen on gonadotropin-releasing hormone (GnRH) secretion. [] This leads to increased GnRH pulses, stimulating the pituitary gland to release follicle-stimulating hormone (FSH) and luteinizing hormone (LH). [, , ] These hormones then stimulate ovulation by promoting follicle growth and maturation in the ovaries. [, , ]
Q2: What are the downstream effects of this compound citrate on hormone levels?
A2: this compound citrate treatment leads to an increase in serum FSH and LH levels. [, , ] In men, this compound citrate administration was found to increase serum FSH and LH concentrations by 145% and 200%, respectively. [] This increase in gonadotropins stimulates follicular development and estrogen production in women. [, , ]
Q3: What is the molecular formula and weight of this compound citrate?
A3: This information is not provided in the provided research articles. Please consult chemical databases like PubChem or DrugBank for this information.
Q4: Is there any spectroscopic data available for this compound citrate?
A4: The provided research articles focus on the clinical and biological aspects of this compound citrate. For detailed spectroscopic data, please refer to specialized chemical databases or publications focusing on the compound's structural analysis.
Q5: How does this compound citrate perform under various storage conditions?
A5: One study investigated the stability of this compound citrate tablets under forced degradation conditions. [] The results suggested that temperature and humidity play significant roles in the degradation of the drug in tablet form. []
Q6: Does this compound citrate exhibit any catalytic properties?
A6: The provided research articles do not indicate any catalytic properties associated with this compound citrate. Its primary mode of action is through binding to estrogen receptors, not through catalytic activity.
Q7: Have there been any computational studies on this compound citrate?
A7: One study described using virtual screening methods, identifying this compound citrate as a potential inhibitor of mutant IDH1, an enzyme implicated in certain cancers. [] Molecular docking simulations suggested that this compound citrate might bind to the allosteric site of the mutant IDH1 enzyme. []
Q8: How do structural modifications of this compound citrate affect its activity?
A8: One research study explored designing new potential estrogen antagonists by modifying the structure of this compound citrate. [] While specific structural changes and their effects aren't detailed in the abstract, the study suggests that modifications could potentially lead to compounds with a better safety profile and binding affinity to estrogen receptors. []
Q9: What formulation strategies are employed to improve the stability or bioavailability of this compound citrate?
A9: The provided research articles primarily focus on the clinical application of commercially available this compound citrate formulations. For specific details on formulation strategies and stability enhancement techniques, please consult pharmaceutical sciences literature or drug product information leaflets.
Q10: Are there specific SHE regulations regarding the handling or disposal of this compound citrate?
A10: The provided articles do not delve into the specifics of SHE regulations for this compound citrate. Always refer to relevant safety data sheets and regulatory guidelines from environmental and health authorities for proper handling and disposal procedures.
Q11: What are the common animal models used to study this compound citrate?
A12: Rat models have been used to study the effects of this compound citrate. [, , ] One study investigated the impact of this compound citrate on reproductive hormones and laying performance in silky fowl hens. []
Q12: What are the typical outcomes measured in clinical trials evaluating this compound citrate for infertility?
A13: Clinical trials commonly assess ovulation rate, pregnancy rate, multiple pregnancy rate, miscarriage rate, and endometrial thickness. [, , , , , , , , ] They also monitor adverse events and patient acceptability of the treatment. [, ]
Q13: What factors contribute to this compound citrate resistance in some women?
A13: Several factors have been associated with this compound citrate resistance, including:
- Duration of infertility: Infertility of more than three years was linked to a higher risk of resistance. []
- Hirsutism: The presence of excessive hair growth was associated with a higher likelihood of resistance. [, ]
- Polycystic ovary syndrome (PCOS): PCOS itself is a significant factor, and specific features like higher antral follicle count (AFC) and an LH:FSH ratio greater than 1 were linked to resistance. [, ]
Q14: Are there any known long-term effects of this compound citrate use?
A15: While this Q&A focuses on the scientific aspects, it's important to acknowledge that potential long-term effects are a concern. One study highlighted a possible link between this compound citrate use during early pregnancy and an increased risk of hypospadias in male offspring. [] Another study suggested that children conceived with ART, which often involves this compound citrate, may have an increased risk of adverse outcomes, including congenital malformations and imprinted gene disorders. []
Q15: Are there any ongoing research efforts to develop targeted delivery systems for this compound citrate?
A15: The provided research articles do not mention specific drug delivery systems designed for this compound citrate. For information on targeted drug delivery approaches in general, please consult pharmaceutical sciences and drug delivery research publications.
Q16: Are there alternative medications to this compound citrate for ovulation induction?
A27: Yes, letrozole, an aromatase inhibitor, is emerging as a potential alternative for ovulation induction, particularly in women with this compound citrate resistance. [, , ] Some studies suggest that letrozole might be associated with higher live birth rates compared to this compound citrate. []
Q17: Are there any head-to-head comparisons between this compound citrate and letrozole for ovulation induction?
A28: Research directly comparing the two drugs exists. One study investigated the efficacy and safety of this compound citrate capsules versus letrozole in treating PCOS. [] The study found that both medications promoted ovulation, but this compound citrate showed superior efficacy, although it was associated with a higher incidence of adverse reactions. []
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。
![8-chloro-1-methyl-5-(4-methylpiperazin-1-yl)-6-phenyl-[1,2,4]triazolo[4,3-a][1,5]benzodiazepine](/img/structure/B125211.png)
![2'-(((tert-Butoxycarbonyl)amino)methyl)-[1,1'-biphenyl]-2-carboxylic acid](/img/structure/B125220.png)