ドキソルビシン
概要
説明
ドキソルビシンは、アドリアマイシンという商品名でも知られており、乳がん、膀胱がん、カポジ肉腫、リンパ腫、急性リンパ性白血病などのさまざまな種類の癌を治療するために使用される化学療法薬です . ドキソルビシンはアントラサイクリン系薬剤に属し、DNAの機能を阻害することにより、最終的に癌細胞の増殖を抑制します .
2. 製法
合成経路と反応条件: ドキソルビシンは、別のアントラサイクリン系抗生物質であるダウノルビシンから合成されます。合成には、グリコシル化、酸化、メチル化などのいくつかのステップが含まれます。 合成における重要な中間体はダウノルビシンであり、これはヒドロキシル化を受けてドキソルビシンを生成します .
工業生産方法: ドキソルビシンの工業生産は、通常、Streptomyces peucetiusという細菌を用いた発酵によって行われます。発酵プロセスには、ドキソルビシンを単離するための抽出および精製ステップが続きます。 遠隔負荷やPEG化などの高度な技術は、ドキソルビシンリポソームの安定性と有効性を向上させるために使用されます .
作用機序
ドキソルビシンは、DNAにインターカレーションすることにより作用し、DNA複製に関与する酵素であるトポイソメラーゼIIの作用を阻害します。 これにより、活性酸素種の生成とそれに続くDNA損傷が起こり、最終的に細胞死につながります . ドキソルビシンは、細胞死を調節する上で重要な役割を果たすBcl-2/Baxアポトーシス経路など、さまざまな分子標的や経路にも影響を与えます .
類似化合物:
- ダウノルビシン
- エピルビシン
- イダルビシン
- ミトキサントロン
比較: ドキソルビシンは、これらの化合物の中で、さまざまな癌に対する幅広い活性スペクトルとDNAにインターカレーションする能力を持っている点がユニークです。 ダウノルビシンは、ドキソルビシンと構造的に類似していますが、側鎖が異なり、薬物動態に影響を与えます . エピルビシンとイダルビシンもアントラサイクリン系薬剤ですが、毒性プロファイルと臨床応用が異なります . ミトキサントロンは、アントラサイクリン系薬剤ではありませんが、一部の構造的類似性を共有しており、同様の臨床設定で使用されます .
結論として、ドキソルビシンは、科学研究や医学において幅広い応用範囲を持つ重要な化学療法剤です。その独自の作用機序と幅広い活性スペクトルは、癌との闘いにおける重要なツールとなっています。
科学的研究の応用
Doxorubicin has a wide range of scientific research applications, particularly in the fields of chemistry, biology, medicine, and industry. In medicine, it is extensively used as a chemotherapeutic agent to treat various cancers. Research has focused on improving its efficacy and reducing its side effects through the development of novel drug delivery systems such as liposomes, nanoparticles, and polymeric micelles .
In biology, doxorubicin is used to study the mechanisms of DNA damage and repair, as well as the cellular responses to oxidative stress. In chemistry, it serves as a model compound for studying the interactions between small molecules and DNA .
生化学分析
Biochemical Properties
Doxorubicin plays a crucial role in biochemical reactions by interacting with several key biomolecules. It intercalates between DNA base pairs, disrupting the DNA structure and inhibiting the activity of topoisomerase II, an enzyme essential for DNA replication and transcription . This interaction leads to the generation of reactive oxygen species, causing oxidative damage to cellular components . Additionally, doxorubicin binds to various proteins, including histones and transcription factors, further affecting gene expression and cellular function .
Cellular Effects
Doxorubicin exerts profound effects on various types of cells and cellular processes. It induces DNA damage, leading to cell cycle arrest and apoptosis . In cancer cells, doxorubicin triggers the activation of apoptotic pathways, including the Bcl-2/Bax pathway, resulting in programmed cell death . The compound also impairs mitochondrial function, leading to the generation of reactive oxygen species and further promoting cell death . In addition to its cytotoxic effects, doxorubicin influences cell signaling pathways, gene expression, and cellular metabolism, contributing to its overall anticancer activity .
Molecular Mechanism
The molecular mechanism of doxorubicin involves several key processes. Upon entering the cell, doxorubicin intercalates into DNA, disrupting the DNA structure and inhibiting the activity of topoisomerase II . This inhibition prevents the relaxation of supercoiled DNA, leading to the accumulation of DNA breaks and ultimately cell death . Doxorubicin also generates reactive oxygen species, causing oxidative damage to cellular components, including lipids, proteins, and DNA . Furthermore, doxorubicin induces the activation of apoptotic pathways, including the Bcl-2/Bax pathway, resulting in programmed cell death .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of doxorubicin change over time. The compound is relatively stable, but its efficacy can decrease due to degradation and the development of drug resistance . Long-term exposure to doxorubicin can lead to chronic cardiotoxicity, characterized by mitochondrial dysfunction and oxidative stress . In vitro and in vivo studies have shown that doxorubicin’s cytotoxic effects are time-dependent, with prolonged exposure leading to increased cell death and tissue damage .
Dosage Effects in Animal Models
The effects of doxorubicin vary with different dosages in animal models. At low doses, doxorubicin induces apoptosis in cancer cells without causing significant toxicity to normal tissues . At high doses, doxorubicin can cause severe cardiotoxicity, characterized by mitochondrial damage, oxidative stress, and cell death . Threshold effects have been observed, with a narrow therapeutic window between effective and toxic doses . Studies in animal models have highlighted the importance of dose optimization to minimize adverse effects while maintaining anticancer efficacy .
Metabolic Pathways
Doxorubicin is involved in several metabolic pathways, including one-electron reduction, two-electron reduction, and deglycosidation . The compound is metabolized by enzymes such as cytochrome P450 reductase and aldo-keto reductase, leading to the formation of active and inactive metabolites . These metabolic pathways influence the pharmacokinetics and toxicity of doxorubicin, affecting its overall efficacy and safety . Additionally, doxorubicin can alter metabolic flux and metabolite levels, further impacting cellular function .
Transport and Distribution
Doxorubicin is transported and distributed within cells and tissues through various mechanisms. The compound is taken up by cells via passive diffusion and active transport, involving transporters such as P-glycoprotein . Once inside the cell, doxorubicin is distributed to different organelles, including the nucleus, mitochondria, and lysosomes . The localization and accumulation of doxorubicin within specific cellular compartments influence its cytotoxic effects and overall efficacy .
Subcellular Localization
The subcellular localization of doxorubicin plays a critical role in its activity and function. Doxorubicin primarily localizes to the nucleus, where it intercalates into DNA and inhibits topoisomerase II . Additionally, doxorubicin accumulates in mitochondria, leading to mitochondrial dysfunction and the generation of reactive oxygen species . The compound can also be found in lysosomes, where it contributes to lysosomal membrane permeabilization and cell death . Targeting signals and post-translational modifications may direct doxorubicin to specific compartments, influencing its subcellular distribution and activity .
準備方法
Synthetic Routes and Reaction Conditions: Doxorubicin is synthesized from daunorubicin, another anthracycline antibiotic. The synthesis involves several steps, including glycosylation, oxidation, and methylation. The key intermediate in the synthesis is daunorubicin, which undergoes hydroxylation to form doxorubicin .
Industrial Production Methods: Industrial production of doxorubicin typically involves fermentation using the bacterium Streptomyces peucetius. The fermentation process is followed by extraction and purification steps to isolate doxorubicin. Advanced techniques such as remote loading and PEGylation are used to enhance the stability and efficacy of doxorubicin liposomes .
化学反応の分析
反応の種類: ドキソルビシンは、酸化、還元、置換など、さまざまな化学反応を起こします。 ドキソルビシンは、DNA関連酵素に結合し、DNA塩基対にインターカレーションすることが知られており、DNA損傷を引き起こします .
一般的な試薬と条件: ドキソルビシンを含む反応で使用される一般的な試薬には、酸化剤、還元剤、さまざまな溶媒が含まれます。 これらの反応の条件は、通常、化合物の安定性を確保するために、制御された温度とpHレベルを伴います .
生成される主要な生成物: ドキソルビシンの反応から生成される主要な生成物には、その代謝産物が含まれ、これらは尿や便を通して排泄されます。 これらの代謝産物は、ヒドロキシル化やグリコシル化などのプロセスを通じて形成されます .
4. 科学研究への応用
ドキソルビシンは、化学、生物学、医学、産業など、さまざまな科学研究分野で幅広い応用範囲を持っています。医学では、ドキソルビシンはさまざまな癌を治療するための化学療法剤として広く使用されています。 研究は、リポソーム、ナノ粒子、高分子ミセルなどの新規な薬物送達システムの開発を通じて、ドキソルビシンの有効性を向上させ、副作用を軽減することに重点を置いてきました .
生物学では、ドキソルビシンは、DNAの損傷と修復のメカニズム、ならびに酸化ストレスに対する細胞の応答を研究するために使用されます。 化学では、ドキソルビシンは、小分子とDNAの相互作用を研究するためのモデル化合物として役立ちます .
類似化合物との比較
- Daunorubicin
- Epirubicin
- Idarubicin
- Mitoxantrone
Comparison: Doxorubicin is unique among these compounds due to its broad spectrum of activity against various cancers and its ability to intercalate with DNA. While daunorubicin is structurally similar to doxorubicin, it has a different side chain, which affects its pharmacokinetic properties . Epirubicin and idarubicin are also anthracyclines but have different toxicity profiles and clinical applications . Mitoxantrone, although not an anthracycline, shares some structural similarities and is used in similar clinical settings .
特性
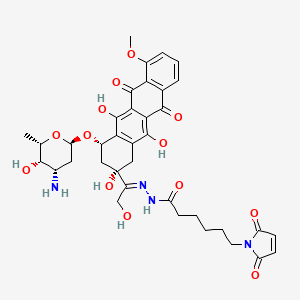
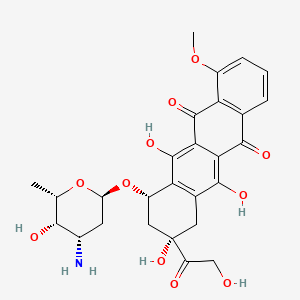
IUPAC Name |
(7S,9S)-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C27H29NO11/c1-10-22(31)13(28)6-17(38-10)39-15-8-27(36,16(30)9-29)7-12-19(15)26(35)21-20(24(12)33)23(32)11-4-3-5-14(37-2)18(11)25(21)34/h3-5,10,13,15,17,22,29,31,33,35-36H,6-9,28H2,1-2H3/t10-,13-,15-,17-,22+,27-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
AOJJSUZBOXZQNB-TZSSRYMLSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1C(C(CC(O1)OC2CC(CC3=C2C(=C4C(=C3O)C(=O)C5=C(C4=O)C(=CC=C5)OC)O)(C(=O)CO)O)N)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C[C@H]1[C@H]([C@H](C[C@@H](O1)O[C@H]2C[C@@](CC3=C2C(=C4C(=C3O)C(=O)C5=C(C4=O)C(=CC=C5)OC)O)(C(=O)CO)O)N)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C27H29NO11 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
25316-40-9 (hydrochloride) | |
| Record name | Doxorubicin [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0023214928 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID8021480 | |
| Record name | Doxorubicin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8021480 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
543.5 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Doxorubicin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015132 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Soluble, 2% sol in water; soluble in aqueous alcohols; moderately soluble in anhydrous methanol; insoluble in non-polar organic solvents, 1.18e+00 g/L | |
| Record name | Doxorubicin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00997 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | DOXORUBICIN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3070 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Doxorubicin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015132 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Doxorubicin has antimitotic and cytotoxic activity through a number of proposed mechanisms of action: Doxorubicin forms complexes with DNA by intercalation between base pairs, and it inhibits topoisomerase II activity by stabilizing the DNA-topoisomerase II complex, preventing the religation portion of the ligation-religation reaction that topoisomerase II catalyzes., Doxorubicin hydrochloride is an antineoplastic antibiotic with pharmacologic actions similar to those of daunorubicin. Although the drug has anti-infective properties, its cytotoxicity precludes its use as an anti-infective agent. The precise and/or principal mechanism(s) of the antineoplastic action of doxorubicin is not fully understood. It appears that the cytotoxic effect of the drug results from a complex system of multiple modes of action related to free radical formation secondary to metabolic activation of the doxorubicin by electron reduction, intercalation of the drug into DNA, induction of DNA breaks and chromosomal aberrations, and alterations in cell membranes induced by the drug. Evidence from in vitro studies in cells treated with doxorubicin suggests that apoptosis (programmed cell death) also may be involved in the drug's mechanism of action. These and other mechanisms (chelation of metal ions to produce drug-metal complexes) also may contribute to the cardiotoxic effects of the drug., Doxorubicin undergoes enzymatic 1- and 2-electron reduction to the corresponding semiquinone and dihydroquinone. 7-Deoxyaglycones are formed enzymatically by 1-electron reduction, and the resulting semiquinone free radical reacts with oxygen to produce the hydroxyl radical in a cascade of reactions; this radical may lead to cell death by reacting with DNA, RNA, cell membranes, and proteins. The dihydroquinone that results from 2-electron reduction of doxorubicin also can be formed by the reaction of 2 semiquinones. In the presence of oxygen, dihydroquinone reacts to form hydrogen peroxide, and in its absence, loses its sugar and gives rise to the quinone methide, a monofunctional alkylating agent with low affinity for DNA. The contribution of dihydroquinone and the quinone methide to the cytotoxicity of doxorubicin is unclear. Experimental evidence indicates that doxorubicin forms a complex with DNA by intercalation between base pairs, causing inhibition of DNA synthesis and DNA-dependent RNA synthesis by the resulting template disordering and steric obstruction. Doxorubicin also inhibits protein synthesis. Doxorubicin is active throughout the cell cycle including the interphase., Several anthracycline-induced effects may contribute to the development of cardiotoxicity. In animals, anthracyclines cause a selective inhibition of cardiac muscle gene expression for ?-actin, troponin, myosin light-chain 2, and the M isoform of creatine kinase, which may result in myofibrillar loss associated with anthracycline-induced cardiotoxicity. Other potential causes of anthracycline-induced cardiotoxicity include myocyte damage from calcium overload, altered myocardial adrenergic function, release of vasoactive amines, and proinflammatory cytokines. Limited data indicate that calcium-channel blocking agents (eg, prenylamine) or beta-adrenergic blocking agents may prevent calcium overload ... It has been suggested that the principal cause of anthracycline-induced cardiotoxicity is associated with free radical damage to DNA., Anthracyclines intercalate DNA, chelate metal ions to produce drug-metal complexes, and generate oxygen free radicals via oxidation-reduction reactions. Anthracyclines contain a quinone structure that may undergo reduction via NADPH-dependent reactions to produce a semiquinone free radical that initiates a cascade of oxygen-free radical generation. It appears that the metabolite, doxorubicinol, may be the moiety responsible for cardiotoxic effects, and the heart may be particularly susceptible to free-radical injury because of relatively low antioxidant concentrations. ... Chelation of metal ions, particularly iron, by the drug results in a doxorubicin-metal complex that catalyzes the generation of reactive oxygen free radicals, and the complex is a powerful oxidant that can initiate lipid peroxidation in the absence of oxygen free radicals. This reaction is not blocked by free-radical scavengers, and probably is the principal mechanism of anthracycline-induced cardiotoxicity., The effect of doxorubicin on reactive oxygen metb in rat heart was investigated. It produced oxygen radicals in heart homogenate, sarcoplasmic reticulum, mitochondria, and cytosol, the major sites of cardiac damage. Superoxide prodn in heart sarcosomes and the mitochondrial fraction was incr. Apparently, free radical formation by doxorubicin, which occurs in the same myocardial compartments that are subject to drug-induced tissue injury, may damage the heart by exceeding the oxygen radical detoxifying capacity of cardiac mitochondria and sarcoplasmic reticulum. | |
| Record name | Doxorubicin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00997 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | DOXORUBICIN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3070 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Impurities |
(8S,10S)-10[(3-amino-2,3,6-trideoxy-alpha-levo-lyxo-hexopyranosyl)oxy]-8-(2-bromo-1,1-dimethoxyethyl)-6,8,11-trihydroxy-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione, (8S,10S)-10[(3-amino-2,3,6-trideoxy-alpha-levo-lyxo-hexopyranosyl)oxy]-8-(bromoacetyl)-6,8,11-trihydroxy-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione, Daunorubicin, Doxorubicinone | |
| Record name | DOXORUBICIN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3070 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Red, crystalline solid | |
CAS No. |
23214-92-8 | |
| Record name | Doxorubicin | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=23214-92-8 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Doxorubicin [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0023214928 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Doxorubicin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00997 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Doxorubicin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8021480 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Doxorubicin | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.041.344 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | DOXORUBICIN | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/80168379AG | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | DOXORUBICIN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3070 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Doxorubicin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015132 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
229-231 °C, 230 °C, MELTING POINT: 205 °C WITH DECOMP; PKA: 8.22; ALMOST ODORLESS; HYGROSCOPIC /HYDROCHLORIDE/, 204 - 205 °C | |
| Record name | Doxorubicin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00997 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | DOXORUBICIN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3070 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Doxorubicin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015132 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。


![3-[4-[2-[[6-amino-9-[(2R,3R,4S,5S)-5-(ethylcarbamoyl)-3,4-dihydroxyoxolan-2-yl]purin-2-yl]amino]ethyl]phenyl]propanoic acid;hydrochloride](/img/structure/B1662843.png)