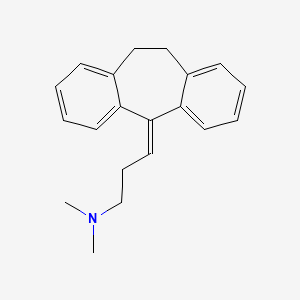
アミトリプチリン
概要
説明
アミトリプチリンは、主に主要なうつ病と、神経性疼痛、線維筋痛症、片頭痛、緊張性頭痛などのさまざまな疼痛症候群の治療に使用される三環系抗うつ薬です . これは、1950年代後半にメルク社の科学者によって発見され、1961年に米国食品医薬品局によって承認されました . アミトリプチリンは、世界保健機関の必須医薬品リストにも掲載されています .
2. 製法
合成ルートと反応条件: アミトリプチリンは、いくつかの方法で合成できます。一般的な方法の1つは、ジベンゾスベロンとジメチルアミンを、水素化リチウムアルミニウムなどの還元剤の存在下で反応させる方法です。 反応は中間体の形成を経て進行し、その後環化してアミトリプチリンが生成されます .
工業的製造方法: 工業的な環境では、アミトリプチリンは通常、高収率と高純度を保証する多段階合成プロセスによって製造されます。 このプロセスには、高性能液体クロマトグラフィー(HPLC)などの高度な技術を使用して精製と品質管理を行うことが含まれます .
作用機序
アミトリプチリンの正確な作用機序は完全に解明されていません。 ノルエピネフリンやセロトニンなどの神経伝達物質の再取り込みを阻害することで、脳内のシナプス間隙におけるこれらの神経伝達物質の濃度を上昇させるものと考えられています . アミトリプチリンは、強力な抗コリン作用も持っており、アルファアドレナリン作動性、ムスカリン作動性、ヒスタミン作動性、ニコチン作動性、NMDA受容体などのさまざまな末梢受容体を遮断することができます .
6. 類似の化合物との比較
アミトリプチリンは、次のような他の三環系抗うつ薬と比較されることがよくあります。
ノルトリプチリン: 構造は似ていますが、副作用は少ないです。
デシプラミン: ノルエピネフリン再取り込み阻害に対する選択性が高いことが知られています。
イミプラミン: 同様の適応症に使用されますが、副作用のプロフィールが異なります。
アミトリプチリンは、複数の神経伝達物質系や受容体に影響を与える幅広いスペクトルの作用により、さまざまな状態の治療に有効であることが特徴です .
科学的研究の応用
Amitriptyline has a wide range of scientific research applications:
Chemistry: Used as a model compound in studies of tricyclic antidepressants.
Biology: Investigated for its effects on neurotransmitter systems and receptor binding.
Industry: Used in the development of new pharmaceutical formulations and drug delivery systems.
生化学分析
Biochemical Properties
Amitriptyline works by increasing the synaptic concentration of serotonin and/or norepinephrine in the central nervous system by inhibiting their reuptake by the presynaptic neuronal membrane pump . This action prolongs the sympathetic activity of these biogenic amines .
Cellular Effects
Amitriptyline has been shown to interfere with the formation of aggresome-like aggregates and autophagy-mediated clearance of aggregates . It also has immunomodulatory effects, such as lowering the production of typical T H 2-cytokines in T lymphocytes of asthmatic mice .
Molecular Mechanism
Amitriptyline’s key mechanism of action lies in the elevation of extracellular biogenic amine levels, notably those of noradrenaline and serotonin, by its blockade of cellular noradrenaline and serotonin reuptake transporters . This response may underlie chronic amitriptyline action on dopamine and norepinephrine neurotransmission .
Temporal Effects in Laboratory Settings
You may have flu-like symptoms like feeling sick, muscle pain, and feeling tired or restless . To help prevent this from happening, your doctor will probably recommend reducing your dose gradually over several weeks .
Dosage Effects in Animal Models
In animal models, oral administration of amitriptyline provides a valid model for behavioral assessment of antidepressant-like effects . The effectiveness of oral amitriptyline varies depending on sex, duration of treatment, and the depression model used .
Metabolic Pathways
Amitriptyline is readily absorbed in the GI tract and subject to extensive hepatic metabolism with less than 5% of the drug eliminated unchanged . The main metabolizing enzymes with clinical significance for amitriptyline are CYP2C19 and CYP2D6 .
Transport and Distribution
Amitriptyline is transported and distributed within cells and tissues via absorption in the GI tract . It is subject to extensive hepatic metabolism .
Subcellular Localization
Given its mechanism of action, it is likely that Amitriptyline localizes to the synapses in neurons where it can inhibit the reuptake of serotonin and norepinephrine .
準備方法
Synthetic Routes and Reaction Conditions: Amitriptyline can be synthesized through several methods. One common method involves the reaction of dibenzosuberone with dimethylamine in the presence of a reducing agent such as lithium aluminum hydride. The reaction proceeds through the formation of an intermediate, which is then cyclized to form amitriptyline .
Industrial Production Methods: In industrial settings, amitriptyline is typically produced through a multi-step synthesis process that ensures high yield and purity. The process involves the use of advanced techniques such as high-performance liquid chromatography (HPLC) for purification and quality control .
化学反応の分析
反応の種類: アミトリプチリンは、以下を含むさまざまな化学反応を起こします。
酸化: アミトリプチリンは酸化されてN-オキシド誘導体を形成することができます。
還元: アミトリプチリンの還元は、第二アミンを形成する可能性があります。
一般的な試薬と条件:
酸化: 一般的な酸化剤には、過酸化水素や過酸が含まれます。
還元: 水素化リチウムアルミニウムや水素化ホウ素ナトリウムなどの還元剤が一般的に使用されます。
生成される主な生成物:
酸化: N-オキシド誘導体の形成。
還元: 第二アミンの形成。
置換: 置換アミトリプチリン誘導体の形成.
4. 科学研究への応用
アミトリプチリンは、幅広い科学研究への応用があります。
化学: 三環系抗うつ薬の研究におけるモデル化合物として使用されます。
生物学: 神経伝達物質系と受容体結合への影響について調査されています。
類似化合物との比較
Amitriptyline is often compared with other tricyclic antidepressants such as:
Nortriptyline: Similar in structure but has fewer side effects.
Desipramine: Known for its higher selectivity for norepinephrine reuptake inhibition.
Imipramine: Used for similar indications but has a different side effect profile.
Doxepin: Also used for depression and anxiety but has additional antihistaminic properties
Amitriptyline is unique due to its broad spectrum of action, affecting multiple neurotransmitter systems and receptors, which contributes to its efficacy in treating a variety of conditions .
特性
IUPAC Name |
N,N-dimethyl-3-(2-tricyclo[9.4.0.03,8]pentadeca-1(15),3,5,7,11,13-hexaenylidene)propan-1-amine | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C20H23N/c1-21(2)15-7-12-20-18-10-5-3-8-16(18)13-14-17-9-4-6-11-19(17)20/h3-6,8-12H,7,13-15H2,1-2H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
KRMDCWKBEZIMAB-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CN(C)CCC=C1C2=CC=CC=C2CCC3=CC=CC=C31 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C20H23N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
17086-03-2 (pamoate (2:1)), 30227-34-0 (maleate (1:1)), 549-18-8 (hydrochloride) | |
| Record name | Amitriptyline [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000050486 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID7022594 | |
| Record name | Amitriptyline | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7022594 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
277.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Amitriptyline | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014466 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Boiling Point |
410.26°C (rough estimate) | |
| Record name | Amitriptyline | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00321 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Solubility |
freely soluble in water, In water, 9.71 mg/L at 24 °C, 4.50e-03 g/L | |
| Record name | Amitriptyline | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00321 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | AMITRIPTYLINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3007 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Amitriptyline | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014466 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
The mechanism of action of this drug is not fully elucidated. It is suggested that amitriptyline inhibits the membrane pump mechanism responsible for the re-uptake of transmitter amines, such as norepinephrine and serotonin, thereby increasing their concentration at the synaptic clefts of the brain,. These amines are important in regulating mood. The monoamine hypothesis in depression, one of the oldest hypotheses, postulates that deficiencies of serotonin (5-HT) and/or norepinephrine (NE) neurotransmission in the brain lead to depressive effects. This drug counteracts these mechanisms, and this may be the mechanism of amitriptyline in improving depressive symptoms. Whether its analgesic effects are related to its mood-altering activities or attributable to a different, less obvious pharmacological action (or a combination of both) is unknown., Acute and chronic effects of the antidepressant drugs tranylcypromine, a monoamine oxidase inhibitor, and amitriptyline, a monoamine uptake inhibitor, were studied on beta-adrenergic receptor function in mouse astrocytes in primary cultures. In clinically relevant concentrations, acute administration of either antidepressant drug had a direct inhibitory effect on the binding of the beta-adrenergic ligand dihydroalprenolol and on the isoproterenol-induced accumulation of cyclic AMP. However, in the absence of isoproterenol, these drugs enhanced the formation of cyclic AMP in the astrocytes. Chronic exposure to amitriptyline or tranylcypromine led to a decrease in isoproterenol-induced accumulation of cyclic AMP, and the time course for the development of this phenomenon was similar to that reported for whole brain in vivo. These findings suggest that these antidepressant drugs act as a partial agonists at beta-adrenergic receptors on astrocytes, and that the down-regulation of beta-adrenergic activity that occurs in vivo after chronic administration of antidepressant drugs may, to a large extent, take place in astrocytes and may result from the partial beta-agonist nature of the drugs., Astrocytes play important roles in guiding the construction of the nervous system, controlling extracellular ions and neurotransmitters, and regulating CNS synaptogenesis. Egr-1 is a transcription factor involved in neuronal differentiation and astrocyte cell proliferation. In this study, we investigated whether the tricyclic antidepressant (TCA) amitriptyline induces Egr-1 expression in astrocytes using rat C6 glioma cells as a model. We found that amitriptyline increased the expression of Egr-1 in a dose- and time-dependent manner. The amitriptyline-induced Egr-1 expression was mediated through serum response elements (SREs) in the Egr-1 promoter. SREs were activated by the Ets-domain transcription factor Elk-1 through the ERK and JNK mitogen-activated protein (MAP) kinase pathways. The inhibition of the ERK and JNK MAP kinase signals attenuated amitriptyline-induced transactivation of Gal4-Elk-1 and Egr-1 promoter activity. Our findings suggest that the induction of Egr-1 expression in astrocytes may be required to attain the therapeutic effects of antidepressant drugs., Antidepressants such as serotonin-noradrenaline reuptake inhibitors (SNRIs) and tricyclic antidepressants (TCAs) are frequently used for the management of neuropathic pain. Noradrenaline (NA) and serotonin (5-HT) increase in the spinal cord by reuptake inhibition is considered to be main mechanism of the therapeutic effect of antidepressants in neuropathic pain. In the present study, we examined the analgesic effects of duloxetine (SNRI) and amitriptyline (TCA) in a rat model of neuropathic pain induced by spinal nerve ligation (SNL). Intraperitoneal administration of duloxetine and amitriptyline dose-dependently (3,10 and 30 mg/kg) suppressed hyperalgesia induced by SNL. In vivo microdialysis in the lumbar spinal dorsal horn revealed that NA and 5-HT concentrations increased after intraperitoneal administration of duloxetine and amitriptyline (10 mg/kg, respectively). We further determined NA and 5-HT contents in homogenized samples from the ipsilateral dorsal spinal cord after SNL. Although the NA content in SNL rats 2 weeks after ligation was higher than that in SNL rats 4 weeks after ligation, the analgesic efficacy of duloxetine and amitriptyline was similar between two groups. The present study suggests that NA/5-HT increase in the spinal cord is crucial in the antihyperalgesic effect of duloxetine and amitriptyline. The plastic change of the descending noradrenergic system does not obviously affect the analgesic efficacy of duloxetine and amitriptyline., Recent studies show that neuronal and glial plasticity are important for the therapeutic action of antidepressants. Here, we demonstrated that amitriptyline, a tricyclic antidepressant, significantly increased GDNF mRNA and GDNF release in C6 cells. Furthermore, different classes of antidepressants increased GDNF release, but non-antidepressant psychotropic drugs did not. The amitriptyline-induced GDNF release was completely inhibited by U0126, a mitogen-activated protein kinase (MAPK)-extracellular signal-regulated kinase (ERK) kinase (MEK) inhibitor, but was not inhibited by H-89, a protein kinase A inhibitor or calphostin C, a protein kinase C inhibitor. These results suggest that the amitriptyline-induced GDNF release may be regulated through a MEK/MAPK pathway. Next, we examined the effects of monoamines on GDNF release, because antidepressants are known to increase monoamines. 5-HT increased GDNF mRNA and GDNF release, but noradrenaline and dopamine did not. The 5-HT-induced GDNF release was partially, but significantly, blocked by ketanserin, a 5-HT2A receptor antagonist. The 5-HT-induced GDNF release was completely inhibited by U0126, but was not inhibited by H-89 or calphostin C. These results suggest that the 5-HT-induced GDNF release was mediated through a MEK/MAPK pathway and, at least, 5-HT2A receptors. GDNF, as well as other neurotrophic factors, may contribute to explain the therapeutic action of antidepressants and suggest a novel strategy of pharmacological intervention., For more Mechanism of Action (Complete) data for AMITRIPTYLINE (13 total), please visit the HSDB record page. | |
| Record name | Amitriptyline | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00321 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | AMITRIPTYLINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3007 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Crystals | |
CAS No. |
50-48-6 | |
| Record name | Amitriptyline | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=50-48-6 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Amitriptyline [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000050486 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Amitriptyline | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00321 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Amitriptyline | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7022594 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Amitriptyline | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.000.038 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | AMITRIPTYLINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/1806D8D52K | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | AMITRIPTYLINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3007 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Amitriptyline | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014466 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
196-197, 196 - 197 °C | |
| Record name | Amitriptyline | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00321 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Amitriptyline | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014466 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods I
Procedure details








Synthesis routes and methods II
Procedure details






Synthesis routes and methods III
Procedure details





Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。

![N-(Methylsulfonyl)-D-Phenylalanyl-N-[(1-Carbamimidoylpiperidin-4-Yl)methyl]-L-Prolinamide](/img/structure/B1667174.png)