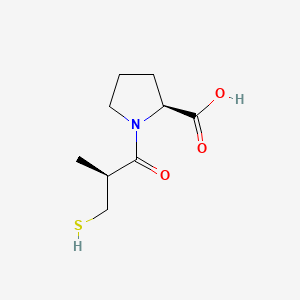
カプトプリル
概要
説明
カプトプリルは、アンジオテンシンIをアンジオテンシンIIに変換する役割を担う、アンジオテンシン変換酵素(ACE)の強力な競合阻害剤です。アンジオテンシンIIは血圧の主要な調節因子であり、レニン・アンジオテンシン・アルドステロン系(RAAS)の重要な構成要素です。 カプトプリルは主に、本態性高血圧または腎血管性高血圧、うっ血性心不全、心筋梗塞後の左室機能不全、および腎症の治療に使用されます .
作用機序
カプトプリルは、アンジオテンシン変換酵素を阻害することで効果を発揮し、アンジオテンシンIからアンジオテンシンIIへの変換を防ぎます。これにより、血管拡張、血圧低下、心臓への負担軽減が起こります。 アンジオテンシンII形成の阻害は、アルドステロンの放出も抑制し、ナトリウムと水の貯留減少につながります .
科学的研究の応用
Captopril has a wide range of scientific research applications. In chemistry, it is used as a model compound for studying enzyme inhibition and reaction mechanisms. In biology, captopril is used to investigate the role of the renin-angiotensin-aldosterone system in various physiological processes. In medicine, captopril is extensively studied for its therapeutic effects in treating hypertension, heart failure, and diabetic nephropathy .
生化学分析
Biochemical Properties
Captopril plays a crucial role in biochemical reactions by inhibiting the angiotensin-converting enzyme (ACE). This enzyme is responsible for converting angiotensin I to angiotensin II, a potent vasoconstrictor. By inhibiting ACE, captopril reduces the levels of angiotensin II, leading to vasodilation and decreased blood pressure . Captopril interacts with several biomolecules, including ACE, bradykinin, and prostaglandins. The interaction with ACE involves the binding of captopril’s thiol group to the zinc ion at the active site of the enzyme, thereby inhibiting its activity .
Cellular Effects
Captopril has significant effects on various cell types and cellular processes. It influences cell function by modulating cell signaling pathways, gene expression, and cellular metabolism. For instance, captopril has been shown to increase cell viability and reduce oxidative stress in C6 cells by decreasing levels of inflammatory markers such as NF-kB, IL-1β, COX-1, and COX-2 . Additionally, captopril can affect the renin-angiotensin-aldosterone system (RAAS), leading to changes in gene expression related to blood pressure regulation and fluid balance .
Molecular Mechanism
The molecular mechanism of captopril involves its competitive inhibition of the angiotensin-converting enzyme (ACE). Captopril binds to the active site of ACE, preventing the conversion of angiotensin I to angiotensin II . This inhibition results in decreased vasoconstriction and reduced aldosterone secretion, leading to lower blood pressure. Furthermore, captopril prevents the degradation of vasodilatory prostaglandins, promoting systemic vasodilation .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of captopril can change over time. Captopril is known to have a relatively short half-life of approximately 2 hours . Its stability and degradation can influence its long-term effects on cellular function. Studies have shown that captopril can reduce inflammation and oxidative stress over extended periods, contributing to its protective effects in conditions such as radiation-induced lung injury .
Dosage Effects in Animal Models
The effects of captopril vary with different dosages in animal models. At therapeutic doses, captopril effectively reduces blood pressure and improves cardiac function . At higher doses, captopril can cause adverse effects such as hypotension, renal impairment, and hyperkalemia . In animal models, captopril has been shown to mitigate radiation-induced lung injury and improve survival rates .
Metabolic Pathways
Captopril is involved in several metabolic pathways, primarily related to the renin-angiotensin-aldosterone system (RAAS). It inhibits the conversion of angiotensin I to angiotensin II, reducing vasoconstriction and aldosterone secretion . Additionally, captopril can influence the kallikrein-kinin-prostaglandin system, affecting prostaglandin production and renal hemodynamics .
Transport and Distribution
Captopril is well-distributed within tissues and is primarily metabolized by the liver . It is excreted unchanged in the urine, with a bioavailability of 70-75% . Captopril binds to plasma proteins and can form mixed disulfides with endogenous thiol-containing compounds such as cysteine and glutathione .
Subcellular Localization
Captopril’s subcellular localization is influenced by its interactions with specific biomolecules and enzymes. It targets the angiotensin-converting enzyme (ACE) located on the surface of endothelial cells . The thiol group of captopril allows it to bind to the active site of ACE, inhibiting its activity and preventing the conversion of angiotensin I to angiotensin II .
準備方法
合成経路と反応条件: カプトプリルはいくつかの方法で合成することができます。一般的な方法の1つは、L-プロリンと2-メチル-3-メルカプトプロピオニルクロリドをトリエチルアミンなどの塩基の存在下で反応させる方法です。 この反応は通常、ジクロロメタンなどの有機溶媒中で低温で行われ、副反応を防ぎます .
工業的製造方法: カプトプリルの工業的製造では、反応条件を厳密に制御して高収率と高純度を確保する、大型反応器の使用が一般的です。このプロセスには、最終生成物を精製するための再結晶化などの工程が含まれます。 連続フロー反応器などの高度な技術を使用すると、製造プロセスの効率とスケーラビリティを向上させることができます .
化学反応の分析
反応の種類: カプトプリルは、酸化、還元、置換など、さまざまな化学反応を起こします。 たとえば、カプトプリルのチオール基は酸化されてジスルフィドを形成することができます。一方、カルボキシル基はエステル化反応に関与することができます .
一般的な試薬と条件: カプトプリルを含む反応で使用される一般的な試薬には、酸化反応用の過酸化水素などの酸化剤と、還元反応用の水素化ホウ素ナトリウムなどの還元剤があります。 反応は通常、化合物の分解を防ぐために、穏やかな条件下で行われます .
生成される主な生成物: これらの反応から生成される主な生成物には、酸化によるカプトプリルジスルフィドと、エステル化反応によるさまざまなエステルが含まれます。 これらの誘導体は、異なる薬理学的特性を持つことがあり、その潜在的な治療用途について研究されています .
科学研究への応用
カプトプリルは、幅広い科学研究に利用されています。化学分野では、酵素阻害と反応機構を研究するためのモデル化合物として使用されます。生物学分野では、カプトプリルはさまざまな生理学的プロセスにおけるレニン・アンジオテンシン・アルドステロン系の役割を調査するために使用されています。 医学分野では、カプトプリルは高血圧、心不全、糖尿病性腎症の治療における治療効果について広く研究されています .
類似化合物との比較
カプトプリルは、エナラプリル、リシノプリル、ラミプリルなどの他のACE阻害剤と比較されることがよくあります。これらの化合物はすべて同じ酵素を阻害しますが、カプトプリルは、その高い効力と急速な作用開始に寄与するチオール基が特徴です。 このチオール基は、皮膚の発疹や味覚障害などの副作用を引き起こす可能性がありますが、これらの副作用は他のACE阻害剤ではそれほど一般的ではありません .
類似化合物のリスト:- エナラプリル
- リシノプリル
- ラミプリル
- ベナゼプリル
カプトプリルの独特の構造と急速な作用は、潜在的な副作用があるものの、貴重な治療薬となっています。 カプトプリルの発見と開発は、心臓血管疾患の治療を大きく進歩させました .
特性
IUPAC Name |
(2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolidine-2-carboxylic acid | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
FAKRSMQSSFJEIM-RQJHMYQMSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(CS)C(=O)N1CCCC1C(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C[C@H](CS)C(=O)N1CCC[C@H]1C(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C9H15NO3S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
1253948-36-5 | |
| Record name | L-Proline, 1-[(2S)-3-mercapto-2-methyl-1-oxopropyl]-, homopolymer | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=1253948-36-5 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
DSSTOX Substance ID |
DTXSID1037197 | |
| Record name | Captopril | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID1037197 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
217.29 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Captopril | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015328 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Freely soluble, Freely soluble in water (approximately 160 mg/mL), Freely soluble in alcohol, chloroform, methylene chloride; sparingly soluble in ethyl acetate, 4.52e+00 g/L | |
| Record name | Captopril | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01197 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Captopril | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6527 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Captopril | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015328 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
There are two isoforms of ACE: the somatic isoform, which exists as a glycoprotein comprised of a single polypeptide chain of 1277; and the testicular isoform, which has a lower molecular mass and is thought to play a role in sperm maturation and binding of sperm to the oviduct epithelium. Somatic ACE has two functionally active domains, N and C, which arise from tandem gene duplication. Although the two domains have high sequence similarity, they play distinct physiological roles. The C-domain is predominantly involved in blood pressure regulation while the N-domain plays a role in hematopoietic stem cell differentiation and proliferation. ACE inhibitors bind to and inhibit the activity of both domains, but have much greater affinity for and inhibitory activity against the C-domain. Captopril, one of the few ACE inhibitors that is not a prodrug, competes with ATI for binding to ACE and inhibits and enzymatic proteolysis of ATI to ATII. Decreasing ATII levels in the body decreases blood pressure by inhibiting the pressor effects of ATII as described in the Pharmacology section above. Captopril also causes an increase in plasma renin activity likely due to a loss of feedback inhibition mediated by ATII on the release of renin and/or stimulation of reflex mechanisms via baroreceptors. Captopril’s affinity for ACE is approximately 30,000 times greater than that of ATI., The local role of the renin angiotensin system (RAS) was documented recently beside its conventional systemic functions. Studies showed that the effector angiotensin II (AngII) alters bone health, while inhibition of the angiotensin converting enzyme (ACE-1) preserved these effects. The newly identified Ang1-7 exerts numerous beneficial effects opposing the AngII. Thus, the current study examines the role of Ang1-7 in mediating the osteo-preservative effects of ACEI (captopril) through the G-protein coupled Mas receptor using an ovariectomized (OVX) rat model of osteoporosis. 8 weeks after the surgical procedures, captopril was administered orally (40 mg/kg/day), while the specific Mas receptor blocker (A-779) was delivered at infusion rate of 400 ng/kg/1 min for 6 weeks. Bone metabolic markers were measured in serum and urine. Minerals concentrations were quantified in serum, urine and femoral bones by inductive coupled plasma mass spectroscopy (ICP-MS). Trabecular and cortical morphometry was analyzed in the right distal femurs using micro-CT. Finally, the expressions of RAS peptides, enzymes and receptors along with the receptor activator of NF-kappaB ligand (RANKL) and osteoprotegerin (OPG) were determined femurs heads. OVX animals markedly showed altered bone metabolism and mineralization along with disturbed bone micro-structure. Captopril significantly restored the metabolic bone bio-markers and corrected Ca2+ and P values in urine and bones of estrogen deficient rats. Moreover, the trabecular and cortical morphometric features were repaired by captopril in OVX groups. Captopril also improved the expressions of ACE-2, Ang1-7, Mas and OPG, while abolished OVX-induced up-regulation of ACE-1, AngII, Ang type 1 receptor (AT1R) and RANKL. Inhibition of Ang1-7 cascade by A-779 significantly eradicated captopril protective effects on bone metabolism, mineralization and micro-structure. A-779 also restored OVX effects on RANKL expression and ACE-1/AngII/AT1R cascade and down-regulated OPG expression and ACE-2/Ang1-7/Mas pathway. In line with the clinical observations of the bone-preservative properties following ACE-1 inhibition, local activation of ACE-2/Ang1-7/Mas signaling and suppressed osteoclastogenesis seem responsible for the osteo-preservative effect of captopril, which could offers a potential therapeutic value in treatment of disabling bone and skeletal muscular diseases. | |
| Record name | Captopril | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01197 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Captopril | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6527 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
White to off-white, crystalline powder, Crystals from ethyl acetate/hexane | |
CAS No. |
62571-86-2 | |
| Record name | Captopril | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=62571-86-2 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Captopril [USAN:USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0062571862 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Captopril | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01197 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | captopril | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=757419 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Captopril | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID1037197 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Captopril | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.057.806 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | CAPTOPRIL | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/9G64RSX1XD | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Captopril | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6527 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Captopril | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015328 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
103-104, 103-104 °C, 106 °C | |
| Record name | Captopril | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01197 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Captopril | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6527 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Captopril | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015328 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods
Procedure details








Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary mechanism of action of Captopril?
A1: Captopril exerts its primary antihypertensive effect by inhibiting the angiotensin-converting enzyme (ACE). [, , , , , , , ] This enzyme catalyzes the conversion of angiotensin I to angiotensin II, a potent vasoconstrictor. By blocking this conversion, Captopril reduces vasoconstriction, leading to a decrease in blood pressure. [, , , , , ]
Q2: Does Captopril impact the renin-angiotensin system (RAS) beyond ACE inhibition?
A2: Yes, research suggests that Captopril can influence the RAS in various ways. For instance, it has been shown to increase plasma renin activity (PRA), likely as a compensatory response to reduced angiotensin II levels. [, , , , ] Additionally, chronic Captopril treatment can lead to changes in the expression of components of the tissue RAS, such as angiotensinogen in the kidneys. []
Q3: How does Captopril impact the nervous system in the context of hypertension?
A4: Studies in spontaneously hypertensive rats (SHR) have shown that chronic Captopril treatment can alter the properties of cardiovascular neurons in the rostral ventrolateral medulla (RVL), a brainstem region involved in blood pressure regulation. [] These changes appear to contribute to the long-term blood pressure-lowering effects of Captopril. []
Q4: Can Captopril cross the blood-brain barrier and directly affect the central nervous system?
A5: While Captopril's ability to cross the blood-brain barrier is limited, research in pregnant sheep suggests that it might cross the placenta and directly influence the fetal renin-angiotensin system. [] This finding highlights the complex interplay between Captopril and the RAS in different physiological contexts.
Q5: What is the molecular formula and weight of Captopril?
A5: Captopril has the molecular formula C9H15NO3S and a molecular weight of 217.29 g/mol.
Q6: Is there information available regarding the spectroscopic data or material compatibility of Captopril within the provided research?
A6: The provided research focuses primarily on the pharmacological and physiological effects of Captopril. It does not delve into detailed spectroscopic data or material compatibility assessments.
Q7: How is Captopril absorbed and metabolized in the body?
A8: Captopril is rapidly absorbed after oral administration, reaching peak plasma concentrations within approximately 1 hour. [, ] It is primarily metabolized in the liver, forming several metabolites, including Captopril-cysteine disulfide. []
Q8: What is the elimination half-life of Captopril?
A9: The elimination half-life of Captopril is relatively short, ranging from approximately 0.7 to 2 hours. [, ] This short half-life necessitates multiple daily doses to maintain therapeutic effects in most patients. [, ]
Q9: How is Captopril's efficacy assessed in preclinical and clinical settings?
A11: Preclinical studies often utilize animal models, such as spontaneously hypertensive rats (SHR), to investigate Captopril's effects on blood pressure, organ damage, and other physiological parameters. [, , , , ] Clinical trials typically assess Captopril's efficacy in humans with hypertension by monitoring blood pressure changes, 24-hour ambulatory blood pressure monitoring, and evaluating its impact on cardiovascular outcomes. [, , , , , ]
Q10: Does Captopril exhibit any direct effects on blood vessels?
A12: Research suggests that Captopril might exert direct effects on blood vessels. For instance, studies in atherosclerotic rabbits demonstrated that Captopril, as well as the angiotensin II type 1 receptor blocker Valsartan, could inhibit the thickening of the abdominal aorta. []
Q11: Does Captopril influence cell proliferation or apoptosis?
A13: Some evidence points to a potential role of Captopril in modulating cell proliferation and apoptosis. A study in spontaneously hypertensive rats found that Captopril treatment could reverse the increased expression of the pro-apoptotic protein Bax and the decreased expression of the anti-apoptotic protein Bcl-2 in capillary endothelial cells of the heart and brain. [] These findings suggest that Captopril may exert protective effects on the vasculature, potentially influencing cell survival pathways.
Q12: Can Captopril impact other organ systems beyond the cardiovascular system?
A14: Yes, research indicates that Captopril might have effects beyond blood pressure regulation. For example, it has been explored for its potential to improve wound healing in diabetic rats, possibly by increasing nitric oxide and vascular endothelial growth factor (VEGF) levels in wound fluid. []
Q13: What are the common side effects associated with Captopril?
A13: While this document focuses on the scientific aspects of Captopril, it is essential to acknowledge that side effects can occur with its use. Please consult relevant clinical resources for comprehensive information on this aspect.
Q14: Are there any specific drug delivery systems or targeting strategies being investigated for Captopril?
A14: The provided research primarily focuses on traditional oral administration of Captopril. While targeted drug delivery holds potential for enhancing efficacy and minimizing off-target effects, the provided literature does not delve into specific strategies for Captopril.
Q15: What analytical methods are commonly used to measure Captopril levels?
A18: Various analytical techniques can be employed to quantify Captopril levels in biological samples. The provided research mentions high-pressure liquid chromatography coupled with mass spectrometry (LC-MS/MS) as a sensitive and specific method for measuring Captopril concentrations in plasma. []
Q16: What were some of the key milestones in the development and use of Captopril?
A20: Captopril was the first clinically approved ACE inhibitor, representing a significant advancement in the treatment of hypertension. [] Its development stemmed from research on peptides isolated from the venom of the Brazilian pit viper Bothrops jararaca, which were found to inhibit ACE. This discovery paved the way for the development of a new class of antihypertensive drugs that revolutionized cardiovascular medicine.
Q17: Does Captopril find applications beyond its primary use in hypertension?
A17: Yes, in addition to its well-established role in hypertension management, Captopril has been investigated for its potential in other areas, including:
- Heart Failure: Captopril has been shown to improve symptoms, reduce hospitalization rates, and enhance survival in patients with heart failure. [, , ]
- Diabetic Nephropathy: Studies suggest that Captopril can slow the progression of kidney disease in individuals with diabetes. []
- Myocardial Infarction: Research indicates that early administration of Captopril after a heart attack can improve long-term survival and reduce the risk of heart failure. [, ]
- Pulmonary Hypertension: Although less commonly used, Captopril has been explored as a treatment option for certain types of pulmonary hypertension. []
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。
![S-[(2R,3S,4S,6S)-6-[[(2R,3S,4S,5R,6R)-5-[(2S,4S,5S)-5-(ethylamino)-4-methoxyoxan-2-yl]oxy-4-hydroxy-6-[[(2S,5Z,9R,13Z)-9-hydroxy-12-(methoxycarbonylamino)-13-[2-(methyltrisulfanyl)ethylidene]-11-oxo-2-bicyclo[7.3.1]trideca-1(12),5-dien-3,7-diynyl]oxy]-2-methyloxan-3-yl]amino]oxy-4-hydroxy-2-methyloxan-3-yl] 4-[(2S,3R,4R,5S,6S)-3,5-dihydroxy-4-methoxy-6-methyloxan-2-yl]oxy-5-iodo-2,3-dimethoxy-6-methylbenzenecarbothioate](/img/structure/B1668231.png)