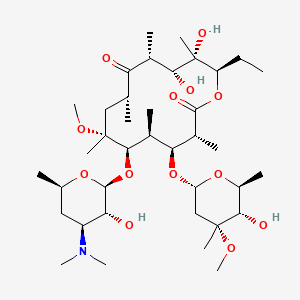
クラリスロマイシン
概要
説明
クラリスロマイシンは、エリスロマイシンから誘導されたマクロライド系抗生物質です。 これは、扁桃腺炎、肺炎、皮膚感染症、ヘリコバクター・ピロリ感染症、ライム病など、様々な細菌感染症の治療に広く用いられています . クラリスロマイシンは、細菌のタンパク質合成を阻害することで作用し、幅広い細菌感染症に対する効果的な治療薬となります .
作用機序
クラリスロマイシンは、細菌の50Sリボソームサブユニットに結合することによりその効果を発揮します。 この結合は、ペプチジル転移酵素活性を阻害し、翻訳およびタンパク質集合過程におけるアミノ酸のトランスロケーションを妨げます。 結果として、細菌のタンパク質合成が阻害され、細菌の増殖が減少し、最終的に体の免疫システムが感染を排除するのを助けます .
科学的研究の応用
Clarithromycin has numerous scientific research applications:
Chemistry: Used as a model compound in studying macrolide antibiotics.
Biology: Investigated for its effects on bacterial protein synthesis.
Industry: Used in the pharmaceutical industry for the production of antibiotics.
生化学分析
Biochemical Properties
Clarithromycin is well absorbed from the gastrointestinal tract and its systemic bioavailability (about 55%) is reduced because of first-pass metabolism . It undergoes rapid biodegradation to produce the microbiologically active 14-hydroxy-®-metabolite .
Cellular Effects
Clarithromycin is indicated for the treatment of bacterial infection associated with sinusitis, tonsillitis, pneumonia, acne (vulgari), and the advanced stage of HIV infections in AIDS patients . In combination with amoxicillin and a proton inhibitor drug, it is used effectively in duodenal ulcer treatment to eradicate helicobacter pylori in a short period of time .
Molecular Mechanism
The main metabolic pathways of Clarithromycin are oxidative N-demethylation and hydroxylation, which are saturable and result in nonlinear pharmacokinetics . The primary metabolite (14-hydroxy derivative) is mainly excreted in the urine with the parent compound .
Temporal Effects in Laboratory Settings
A selective, sensitive, and stability-indicating reversed-phase high-performance liquid chromatography method was developed and validated for the determination of Clarithromycin antibiotic in human plasma . Stock solutions and calibration standards of the drug and quality control preparations were demonstrated to be stable at room temperature and –20°C for long and short periods of time .
Metabolic Pathways
Clarithromycin is involved in metabolic pathways that include oxidative N-demethylation and hydroxylation . The primary metabolite (14-hydroxy derivative) is mainly excreted in the urine with the parent compound .
Transport and Distribution
Clarithromycin is well distributed throughout the body and achieves higher concentrations in tissues than in the blood . Also, the 14-hydroxy metabolite exhibits high tissue concentrations, with values about one-third of the parent compound concentrations .
準備方法
クラリスロマイシンは、一連の化学反応によってエリスロマイシンから合成されます。 このプロセスには、オキシム化、エーテル化、シリル化、メチル化、還元加水分解が含まれます . クラリスロマイシンの工業生産は、一般的に以下の手順で行われます。
オキシム化反応: エリスロマイシンチオシアン酸塩は、オキシム化反応を受けます。
エーテル化反応: オキシムは、エーテル化されます。
シリル化反応: エーテル化された生成物は、シリル化されます。
メチル化反応: シリル化された生成物は、メチル化されます。
還元加水分解: 最後に、生成物は還元され、加水分解されてクラリスロマイシンが得られます.
化学反応の分析
クラリスロマイシンは、以下を含む様々な化学反応を受けます。
酸化: クラリスロマイシンは、特定の条件下で酸化することができます。
還元: 還元反応は、その合成過程の一部です。
これらの反応で使用される一般的な試薬には、有機溶媒、酸、塩基があります。 これらの反応から生成される主な生成物は、最終生成物であるクラリスロマイシンにつながる中間体です .
科学研究への応用
クラリスロマイシンは、多くの科学研究に応用されています。
化学: マクロライド系抗生物質の研究におけるモデル化合物として用いられています。
生物学: 細菌のタンパク質合成に対する効果について調べられています。
類似化合物との比較
クラリスロマイシンは、エリスロマイシンやアジスロマイシンなどの他のマクロライド系抗生物質と似ています。 それは、それを際立たせるユニークな特性を持っています。
エリスロマイシン: クラリスロマイシンは、エリスロマイシンの6-O-メチル誘導体であり、その安定性とバイオアベイラビリティが向上しています.
アジスロマイシン: どちらもマクロライドですが、クラリスロマイシンはより幅広い活性を持ち、特定の細菌株に対してより効果的です.
類似の化合物には以下が含まれます。
- エリスロマイシン
- アジスロマイシン
- アモキシシリン
- オーグメンチン(アモキシシリン/クラブラネート)
クラリスロマイシンのユニークな化学構造と向上した安定性は、様々な細菌感染症の治療において貴重な抗生物質となっています。
特性
IUPAC Name |
(3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-6-[(2S,3R,4S,6R)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-14-ethyl-12,13-dihydroxy-4-[(2R,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyloxan-2-yl]oxy-7-methoxy-3,5,7,9,11,13-hexamethyl-oxacyclotetradecane-2,10-dione | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C38H69NO13/c1-15-26-38(10,45)31(42)21(4)28(40)19(2)17-37(9,47-14)33(52-35-29(41)25(39(11)12)16-20(3)48-35)22(5)30(23(6)34(44)50-26)51-27-18-36(8,46-13)32(43)24(7)49-27/h19-27,29-33,35,41-43,45H,15-18H2,1-14H3/t19-,20-,21+,22+,23-,24+,25+,26-,27+,29-,30+,31-,32+,33-,35+,36-,37-,38-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
AGOYDEPGAOXOCK-KCBOHYOISA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCC1C(C(C(C(=O)C(CC(C(C(C(C(C(=O)O1)C)OC2CC(C(C(O2)C)O)(C)OC)C)OC3C(C(CC(O3)C)N(C)C)O)(C)OC)C)C)O)(C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC[C@@H]1[C@@]([C@@H]([C@H](C(=O)[C@@H](C[C@@]([C@@H]([C@H]([C@@H]([C@H](C(=O)O1)C)O[C@H]2C[C@@]([C@H]([C@@H](O2)C)O)(C)OC)C)O[C@H]3[C@@H]([C@H](C[C@H](O3)C)N(C)C)O)(C)OC)C)C)O)(C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C38H69NO13 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID3022829 | |
| Record name | Clarithromycin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3022829 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
748.0 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Clarithromycin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015342 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
2.17e-01 g/L | |
| Record name | Clarithromycin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01211 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Clarithromycin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015342 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Clarithromycin is first metabolized to 14-OH clarithromycin, which is active and works synergistically with its parent compound. Like other macrolides, it then penetrates bacteria cell wall and reversibly binds to domain V of the 23S ribosomal RNA of the 50S subunit of the bacterial ribosome, blocking translocation of aminoacyl transfer-RNA and polypeptide synthesis. Clarithromycin also inhibits the hepatic microsomal CYP3A4 isoenzyme and P-glycoprotein, an energy-dependent drug efflux pump., Clarithromycin usually is bacteriostatic, although it may be bactericidal in high concentrations or against highly susceptible organisms. Bactericidal activity has been observed against Streptococcus pyogenes, S. pneumoniae, Haemophilus influenzae, and Chlamydia trachomatis. Clarithromycin inhibits protein synthesis in susceptible organisms by penetrating the cell wall and binding to 50S ribosomal subunits, thereby inhibiting translocation of aminoacyl transfer-RNA and inhibiting polypeptide synthesis. The site of action of clarithromycin appears to be the same as that of erythromycin, clindamycin, lincomycin, and chloramphenicol. | |
| Record name | Clarithromycin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01211 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Clarithromycin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8055 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Colorless needles from chloroform + diisopropyl ether (1:2) ... Also reported as crystals from ethanol | |
CAS No. |
81103-11-9, 116836-41-0 | |
| Record name | Clarithromycin | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=81103-11-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Clarithromycin [USAN:USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0081103119 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | (14R)-14-Hydroxyclarithromycin | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0116836410 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Clarithromycin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01211 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | clarithromycin | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=758704 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Clarithromycin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3022829 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Erythromycin, 6-O-methyl | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.119.644 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | Clarithromycin | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | CLARITHROMYCIN | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/H1250JIK0A | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Clarithromycin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8055 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Clarithromycin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015342 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
217-220 °C (decomposes) ... Also reported as mp 222-225 °C, 217 - 220 °C | |
| Record name | Clarithromycin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01211 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Clarithromycin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8055 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Clarithromycin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015342 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary mechanism of action of clarithromycin?
A1: Clarithromycin exerts its antibacterial effect by binding to the 23S ribosomal RNA (rRNA) of susceptible bacteria. [, , , ] This binding interferes with bacterial protein synthesis, ultimately leading to bacterial growth inhibition or death. [, ]
Q2: Why is clarithromycin effective against a wide range of bacterial species?
A2: Clarithromycin exhibits activity against a broad spectrum of bacteria, encompassing both Gram-positive and some Gram-negative species. [] This is largely attributed to the conserved nature of the 23S rRNA target across different bacterial species. []
Q3: Does clarithromycin exhibit bactericidal or bacteriostatic activity?
A3: Clarithromycin's activity can be both bactericidal (killing bacteria) and bacteriostatic (inhibiting bacterial growth) depending on the bacterial species, the concentration of the antibiotic, and the site of infection. [, ] For example, clarithromycin demonstrates bactericidal activity against Haemophilus influenzae and some strains of Mycobacterium avium. [, ]
Q4: How does the activity of clarithromycin's primary metabolite, 14-hydroxyclarithromycin, compare to the parent drug?
A4: 14-hydroxyclarithromycin, the major metabolite of clarithromycin in humans, also possesses antibacterial activity. [, ] Interestingly, this metabolite often exhibits greater potency against certain bacteria, such as Haemophilus influenzae, compared to clarithromycin itself. [, ]
Q5: What is the molecular formula and weight of clarithromycin?
A5: Clarithromycin is represented by the molecular formula C38H69NO13. It has a molecular weight of 747.95 g/mol. [, ]
Q6: How stable is clarithromycin in acidic environments?
A6: Clarithromycin demonstrates instability in low pH solutions. [] Research indicates that its degradation rate increases with decreasing pH. []
Q7: Can the stability of clarithromycin in acidic conditions be improved?
A7: Yes, incorporating polymers like Carbopol 934p and ethylcellulose into clarithromycin formulations provides a protective effect against degradation in low pH environments. [] This suggests that formulation strategies can significantly impact the stability of clarithromycin.
Q8: What is the primary mechanism of resistance to clarithromycin in bacteria like Helicobacter pylori?
A8: The most prevalent mechanism of clarithromycin resistance, particularly in Helicobacter pylori, involves point mutations within the 23S rRNA gene. [, , , , , ] These mutations, often occurring at positions A2142G, A2143G, and A2144G, alter the binding site of clarithromycin, reducing its efficacy. [, , , , ]
Q9: Is there a correlation between the specific 23S rRNA mutation and the level of clarithromycin resistance?
A9: Yes, different point mutations within the 23S rRNA gene can lead to varying degrees of resistance to clarithromycin. [, ] For instance, the A2143G mutation is frequently associated with higher levels of resistance compared to the A2142G mutation. [, ]
Q10: Does clarithromycin resistance impact the effectiveness of combination therapies for Helicobacter pylori eradication?
A10: Yes, the presence of clarithromycin-resistant Helicobacter pylori strains significantly diminishes the success rate of clarithromycin-containing eradication therapies. [, , , , , , ] This highlights the importance of considering antibiotic resistance profiles when selecting treatment regimens.
Q11: How is clarithromycin metabolized in the body?
A11: Clarithromycin undergoes significant first-pass metabolism in the liver, primarily by cytochrome P450 enzymes, leading to the formation of several metabolites, including the microbiologically active 14-hydroxyclarithromycin. [, , ]
Q12: How does the pharmacokinetic profile of clarithromycin change with repeated dosing?
A12: Clarithromycin exhibits non-linear pharmacokinetics, meaning that its elimination half-life and area under the curve (AUC) do not increase proportionally with increasing doses. [] This is thought to be partly due to the saturation of metabolic enzymes involved in its metabolism. []
Q13: Does clarithromycin interact with other drugs that are metabolized by cytochrome P450 enzymes?
A13: Yes, clarithromycin can inhibit the activity of cytochrome P450 3A4, a major drug-metabolizing enzyme. [, ] This inhibition can elevate the plasma concentrations of other drugs metabolized by this enzyme, potentially leading to increased risk of adverse effects. [, ]
Q14: How does clarithromycin's efficacy against MAC infections compare to other antibiotics?
A14: Clarithromycin has shown promise in treating MAC infections, but its efficacy compared to other antibiotics, such as rifampicin and ethambutol, varies depending on the specific MAC species, the severity of infection, and the patient population. [, , , ]
Q15: What are the potential benefits of using liposomal formulations of clarithromycin?
A15: Liposomal formulations of clarithromycin have been explored as a way to enhance its efficacy, particularly against resistant strains of Pseudomonas aeruginosa. [] Encapsulating clarithromycin within liposomes can improve its delivery to target cells and reduce its toxicity. []
Q16: What analytical techniques are commonly employed to determine clarithromycin concentrations in biological samples?
A16: High-performance liquid chromatography (HPLC) coupled with various detection methods, such as UV detection or tandem mass spectrometry (LC-MS/MS), are frequently used to quantify clarithromycin and its metabolites in biological matrices like plasma and serum. [, , , ]
Q17: How is clarithromycin resistance typically assessed in laboratory settings?
A17: Clarithromycin resistance can be assessed through phenotypic methods like the Etest or agar dilution, which measure the minimum inhibitory concentration (MIC) of the antibiotic required to inhibit bacterial growth. [, , , ] Genotypic methods, such as polymerase chain reaction (PCR)-based techniques, are also employed to detect specific mutations in the 23S rRNA gene associated with clarithromycin resistance. [, , , ]
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。