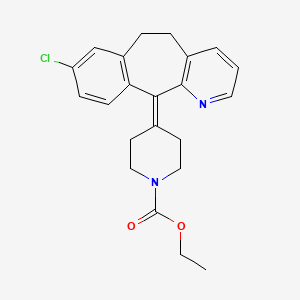
ロラタジン
概要
説明
ロラタジンは、アレルギー性鼻炎やじん麻疹(蕁麻疹)の症状を管理するために広く使用されている第二世代の抗ヒスタミン薬です。 ロラタジンは、くしゃみ、かゆみ、目のかすみなどのアレルギー症状の治療に効果的であり、有意な鎮静作用を引き起こさないため、第一世代の抗ヒスタミン薬よりも好まれます .
作用機序
ロラタジンは、末梢ヒスタミンH1受容体の選択的逆アゴニストとして作用します。アレルギー反応中にヒスタミンが放出されると、これらの受容体に結合し、かゆみやくしゃみなどの症状を引き起こします。 ロラタジンはこの結合をブロックするため、アレルギー反応を効果的に阻止します . ロラタジンは中枢神経系への影響が少なく、鎮静作用のリスクを軽減します .
6. 類似の化合物との比較
ロラタジンは、セチリジンやフェキソフェナジンなどの他の第二世代の抗ヒスタミン薬と比較されることがよくあります。 ジフェンヒドラミンなどの第一世代の抗ヒスタミン薬とは異なり、ロラタジンは血脳関門を有意に通過しないため、鎮静作用が少なくなります . 類似の化合物には以下が含まれます。
セチリジン: アレルギー反応の治療に効果的ですが、軽度の眠気などの副作用を引き起こす可能性があります.
フェキソフェナジン: 作用機序が似ている、もう1つの非鎮静性抗ヒスタミン薬.
クロルフェニラミン: 効果的ですが、有意な鎮静作用を引き起こす、第一世代の抗ヒスタミン薬.
ロラタジンのユニークな利点は、眠気などの副作用を引き起こさずに効果的なアレルギーの緩和を提供できることであり、多くの患者にとって好ましい選択肢となっています。
科学的研究の応用
Loratadine has a wide range of scientific research applications:
生化学分析
Biochemical Properties
Loratadine functions primarily by binding to histamine H1 receptors, which are G-protein coupled receptors located on the surface of various cells, including epithelial cells, endothelial cells, eosinophils, neutrophils, airway cells, and vascular smooth muscle cells . By binding to these receptors, loratadine prevents histamine from exerting its effects, thereby reducing allergic symptoms. The interaction between loratadine and the H1 receptor is characterized by its high affinity and selectivity, which contributes to its effectiveness in blocking histamine-induced responses .
Cellular Effects
Loratadine exerts several effects on different cell types and cellular processes. It has been shown to influence cell signaling pathways, gene expression, and cellular metabolism. For instance, loratadine can modulate the activity of various signaling molecules involved in inflammatory responses, such as cytokines and chemokines . Additionally, loratadine has been associated with improved prognosis in certain cancers, such as lung cancer, by inducing apoptosis and reducing epithelial-mesenchymal transition . These effects are mediated through its interaction with specific cellular receptors and signaling pathways, highlighting its potential therapeutic benefits beyond allergy management.
Molecular Mechanism
At the molecular level, loratadine exerts its effects by acting as an inverse agonist of the histamine H1 receptor . This means that loratadine not only blocks the binding of histamine to the receptor but also stabilizes the receptor in its inactive state, thereby reducing its basal activity. The binding of loratadine to the H1 receptor involves interactions with specific amino acid residues within the receptor’s binding pocket, which prevents the conformational changes required for receptor activation . This mechanism of action underlies the antihistaminic and anti-inflammatory effects of loratadine.
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of loratadine have been studied over various time periods to assess its stability, degradation, and long-term impact on cellular function. Loratadine is known to have a relatively long half-life, which contributes to its sustained therapeutic effects . Studies have shown that loratadine and its active metabolite, desloratadine, can maintain their efficacy over extended periods, with minimal degradation . Additionally, long-term exposure to loratadine has been associated with consistent anti-inflammatory and antihistaminic effects, further supporting its use in chronic allergic conditions .
Dosage Effects in Animal Models
The effects of loratadine at different dosages have been extensively studied in animal models. These studies have revealed that loratadine exhibits dose-dependent effects, with higher doses leading to more pronounced therapeutic outcomes . Excessive dosages can result in adverse effects, such as drowsiness, dry mouth, and gastrointestinal disturbances . In cancer models, moderate concentrations of loratadine have been shown to induce apoptosis and reduce epithelial-mesenchymal transition, while higher concentrations can trigger pyroptosis . These findings highlight the importance of optimizing loratadine dosage to achieve the desired therapeutic effects while minimizing potential side effects.
Metabolic Pathways
Loratadine undergoes extensive first-pass metabolism in the liver, primarily mediated by cytochrome P450 enzymes, including CYP3A4, CYP2D6, CYP1A1, and CYP2C19 . The major metabolite of loratadine is desloratadine, which retains antihistaminic activity and contributes to the overall therapeutic effects of the drug . The metabolic pathways involved in loratadine metabolism also include hydroxylation and conjugation reactions, which facilitate the elimination of the drug from the body . Understanding these metabolic pathways is crucial for optimizing loratadine dosing and minimizing potential drug interactions.
Transport and Distribution
Loratadine is transported and distributed within cells and tissues through various mechanisms. It binds to plasma proteins, which facilitates its distribution throughout the body . The tissue distribution of loratadine and its metabolites has been studied in animal models, revealing that they are widely distributed in organs such as the liver, spleen, thymus, heart, adrenal glands, and pituitary gland . The concentrations of loratadine and its metabolites in these tissues are influenced by factors such as blood flow, tissue permeability, and binding affinity to cellular receptors . These findings provide insights into the pharmacokinetics and tissue-specific effects of loratadine.
Subcellular Localization
The subcellular localization of loratadine and its effects on cellular activity have been investigated to understand its precise mechanism of action. Loratadine is known to localize to specific cellular compartments, such as the plasma membrane and cytoplasm, where it interacts with histamine H1 receptors . The targeting of loratadine to these compartments is facilitated by its chemical structure and binding properties, which enable it to effectively block histamine-induced signaling pathways . Additionally, loratadine may undergo post-translational modifications that influence its localization and activity within cells
準備方法
合成経路と反応条件: ロラタジンはさまざまな方法で合成できます。一般的な方法の1つは、エチル4-(8-クロロ-5,6-ジヒドロ-11H-ベンゾ[5,6]シクロヘプタ[1,2-b]ピリジン-11-イリデン)-1-ピペリジンカルボキシレートを適切な試薬と制御された条件下で反応させることを含みます。 このプロセスは通常、粗製ロラタジンを有機溶媒に溶解し、活性炭を吸着のために添加し、加熱、撹拌、ろ過して精製されたロラタジンを得る手順を含みます .
工業生産方法: 工業用では、ロラタジンは高速せん断高圧ホモジナイザーを用いて製造され、その後凍結乾燥されてロラタジンナノ結晶が生成されます。 この方法はロラタジンの溶解度とバイオアベイラビリティを向上させ、経口投与によりより効果的になります .
化学反応の分析
反応の種類: ロラタジンは、酸化、還元、置換など、さまざまな化学反応を起こします。 例えば、酸化はピペリジン環とシクロヘプタン環で起こる可能性があります .
一般的な試薬と条件: これらの反応で使用される一般的な試薬には、酸化に過酸化水素、還元に水素化ホウ素ナトリウムが含まれます。条件は通常、目的の反応結果を確保するために、制御された温度とpHレベルを伴います。
生成される主な生成物: これらの反応から生成される主な生成物には、ロラタジンの活性代謝物であり、抗ヒスタミン作用を保持するデスロラタジンが含まれます .
4. 科学研究への応用
ロラタジンは、さまざまな科学研究に応用されています。
類似化合物との比較
Loratadine is often compared with other second-generation antihistamines such as cetirizine and fexofenadine. Unlike first-generation antihistamines like diphenhydramine, loratadine does not cross the blood-brain barrier significantly, resulting in fewer sedative effects . Similar compounds include:
Cetirizine: Known for its effectiveness in treating allergic reactions but may cause mild drowsiness.
Fexofenadine: Another non-sedating antihistamine with a similar mechanism of action.
Chlorpheniramine: A first-generation antihistamine that is effective but causes significant sedation.
Loratadine’s unique advantage lies in its ability to provide effective allergy relief without causing drowsiness, making it a preferred choice for many patients.
特性
IUPAC Name |
ethyl 4-(13-chloro-4-azatricyclo[9.4.0.03,8]pentadeca-1(11),3(8),4,6,12,14-hexaen-2-ylidene)piperidine-1-carboxylate | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C22H23ClN2O2/c1-2-27-22(26)25-12-9-15(10-13-25)20-19-8-7-18(23)14-17(19)6-5-16-4-3-11-24-21(16)20/h3-4,7-8,11,14H,2,5-6,9-10,12-13H2,1H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
JCCNYMKQOSZNPW-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCOC(=O)N1CCC(=C2C3=C(CCC4=C2N=CC=C4)C=C(C=C3)Cl)CC1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C22H23ClN2O2 | |
| Record name | loratadine | |
| Source | Wikipedia | |
| URL | https://en.wikipedia.org/wiki/Loratadine | |
| Description | Chemical information link to Wikipedia. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID2023224 | |
| Record name | Loratadine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2023224 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
382.9 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Loratadine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0005000 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
<1 mg/ml at 25°C | |
| Record name | Loratadine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00455 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Mechanism of Action |
Histamine release is a key mediator in allergic rhinitis and urticaria. As a result, loratadine exerts it's effect by targeting H1 histamine receptors. Loratadine binds to H1 histamine receptors found on the surface of epithelial cells, endothelial cells, eosinophils, neutrophils, airway cells, and vascular smooth muscle cells among others. H1 histamine receptors fall under the wider umbrella of G-protein coupled receptors, and exist in a state of equilibrium between the active and inactive forms. Histamine binding to the H1-receptor facilitates cross linking between transmembrane domains III and V, stabilizing the active form of the receptor. On the other hand, antihistamines bind to a different site on the H1 receptor favouring the inactive form. Hence, loratadine can more accurately be classified as an "inverse agonist" as opposed to a "histamine antagonist", and can prevent or reduce the severity of histamine mediated symptoms., All of the available H1 receptor antagonists are reversible, competitive inhibitors of the interaction of histamine with H1 receptors. /H1 Receptor Antagonists/, H1 antagonists inhibit most responses of smooth muscle to histamine. /H1 Antagonists Receptors/, Within the vascular tree, the H1 antagonists inhibit both the vasoconstrictor effects of histamine and, to a degree, the more rapid vasodilator effects that are mediated by H1 receptors on endothelial cells. /H1 Receptor Antagonists/, H1 antagonists strongly block the action of histamine that results in increased capillary permeability and formation of edema and wheal. /H1 Receptor Antagonists/, For more Mechanism of Action (Complete) data for LORATADINE (6 total), please visit the HSDB record page. | |
| Record name | Loratadine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00455 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | LORATADINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3578 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Crystals from acetonitrile | |
CAS No. |
79794-75-5 | |
| Record name | Loratadine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=79794-75-5 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Loratadine [USAN:USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0079794755 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Loratadine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00455 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | loratadine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=758628 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | loratadine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=721075 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Loratadine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2023224 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | ethyl 4-(8-chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)piperidine-1-carboxylate | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.216.235 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | 1-Piperidinecarboxylic acid, 4-(8-chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)-, ethyl ester | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.120.122 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | LORATADINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/7AJO3BO7QN | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | LORATADINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3578 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Loratadine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0005000 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
134-136 °C, 134 - 136 °C | |
| Record name | Loratadine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00455 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | LORATADINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3578 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Loratadine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0005000 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods I
Procedure details









Synthesis routes and methods II
Procedure details








Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。
![(1S,9R,13R,19S,22S,23S,25R,26R,28S,31S,33S,36R)-22-Hydroxy-1,10,10,12,12,30,30,36-octamethyl-28-(2-methylprop-1-enyl)-11,24,27,29,32-pentaoxa-3-azadecacyclo[17.17.0.02,17.04,16.07,15.09,13.022,36.023,25.023,33.026,31]hexatriaconta-2(17),4(16),5,7(15)-tetraen-8-one](/img/structure/B1675035.png)
