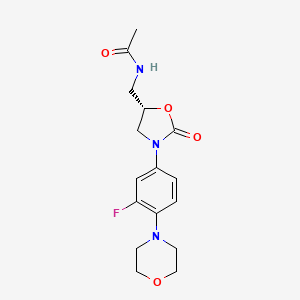
リネゾリド
概要
説明
リネゾリドは、オキサゾリジノン系に属する合成抗生物質です。 主にグラム陽性菌による感染症の治療に使用され、バンコマイシン耐性腸球菌(VRE)やメチシリン耐性黄色ブドウ球菌(MRSA)など、他の抗生物質に耐性を示す菌種に対しても効果を発揮します 。 リネゾリドは、細菌のタンパク質合成を阻害することで作用し、様々な細菌感染症の治療において重要な選択肢となっています .
2. 製法
合成経路と反応条件: リネゾリドは、いくつかの重要な中間体を経る多段階プロセスによって合成されます。 一般的な方法の1つには、® - N - [[3- [3-フルオロ-4-モルフォリニル] フェニル] -2-オキソ-5-オキサゾリジニル] メタノールの調製が含まれ、これはその後、一連の反応を経てリネゾリドに変換されます 。 このプロセスには、通常、置換、環化、アミド化などのステップが含まれます .
工業的製造方法: リネゾリドの工業的製造には、高収率と高純度を達成するために合成経路を最適化することが含まれます。 このプロセスは、費用対効果が高く、環境に優しいように設計されており、廃棄物と副生成物が最小限に抑えられています 。 重要なステップには、金属塩基の使用や、目的の生成物を得るための特定の反応条件があります .
3. 化学反応解析
反応の種類: リネゾリドは、酸化、還元、置換など、様々な化学反応を起こします 。これらの反応は、リネゾリドの合成と修飾に不可欠です。
一般的な試薬と条件: リネゾリドの合成に使用される一般的な試薬には、フタルイミドカリウム、金属塩基、特定の溶媒が含まれます 。温度、圧力、pHなどの反応条件は、最適な結果を得るために慎重に制御されます。
主要な生成物: これらの反応から形成される主要な生成物は、リネゾリドそのものと、® - N - [[3- [3-フルオロ-4-モルフォリニル] フェニル] -2-オキソ-5-オキサゾリジニル] メタノールなどの中間体です .
作用機序
リネゾリドは、細菌のタンパク質合成を阻害することで作用します。 50Sサブユニットの23SリボソームRNAに結合し、細菌翻訳に不可欠な70S開始複合体の形成を阻害します 。 この阻害は、細菌の増殖と複製を停止させ、リネゾリドを幅広いグラム陽性菌に対して効果的なものにします .
類似化合物:
リネゾリドの独自性: リネゾリドは、タンパク質合成の開始を阻害するという、他の抗生物質ではあまり見られない作用機序を持つことで、ユニークです 。 耐性菌に対する有効性と、比較的低い耐性率により、重症感染症の治療における重要な選択肢となっています .
科学的研究の応用
リネゾリドは、様々な科学研究において幅広く応用されています。
化学: 化学分野では、リネゾリドは、その独特な合成経路と、新しい抗生物質開発のためのモデル化合物としての可能性について研究されています .
生物学: 生物学分野では、リネゾリドは、細菌のタンパク質合成と耐性機構の研究に使用されます。 細菌がどのように耐性を発達させるか、そしてこの課題を克服するために新しい抗生物質をどのように設計できるかについての洞察を提供します .
医学: 医学分野では、リネゾリドは、肺炎、皮膚感染症、耐性菌による感染症など、様々な細菌感染症の治療に使用されます 。 また、薬剤耐性結核の治療における可能性についても研究されています .
工業: 製薬業界において、リネゾリドは、重症の細菌感染症の治療に用いられる重要な医薬品です。 リネゾリドの製造と製剤は、研究開発の重要な分野です .
生化学分析
Biochemical Properties
Linezolid plays a crucial role in inhibiting bacterial protein synthesis by binding to the 50S ribosomal subunit . This binding prevents the formation of the initiation complex for protein synthesis, thereby halting bacterial growth . Linezolid interacts with various biomolecules, including ribosomal RNA and proteins involved in the translation process . The nature of these interactions is primarily inhibitory, as Linezolid disrupts the normal function of the ribosome .
Cellular Effects
Linezolid exerts significant effects on bacterial cells by inhibiting protein synthesis, leading to cell death . In mammalian cells, Linezolid can affect mitochondrial protein synthesis due to the similarity between bacterial ribosomes and mitochondrial ribosomes . This can result in mitochondrial dysfunction, impacting cellular metabolism and energy production . Linezolid has also been shown to influence cell signaling pathways and gene expression related to stress responses and apoptosis .
Molecular Mechanism
The molecular mechanism of Linezolid involves its binding to the 50S ribosomal subunit, specifically targeting the peptidyl transferase center . This binding inhibits the formation of the initiation complex, preventing the elongation of the nascent peptide chain . Linezolid’s interaction with ribosomal RNA and proteins is highly specific, leading to the inhibition of protein synthesis . Additionally, Linezolid can induce changes in gene expression related to stress responses and apoptosis .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of Linezolid can change over time due to its stability and degradation . Linezolid is relatively stable, but its long-term effects on cellular function can include mitochondrial dysfunction and altered cellular metabolism . In vitro studies have shown that prolonged exposure to Linezolid can lead to the accumulation of its metabolites, which may contribute to its long-term effects .
Dosage Effects in Animal Models
The effects of Linezolid vary with different dosages in animal models . At therapeutic doses, Linezolid effectively inhibits bacterial growth without causing significant toxicity . At higher doses, Linezolid can cause adverse effects, including hematological toxicity and mitochondrial dysfunction . Threshold effects have been observed, where higher doses lead to more pronounced toxic effects .
Metabolic Pathways
Linezolid is metabolized primarily in the liver, involving oxidation and reduction reactions . The major metabolites of Linezolid are excreted in the urine . Enzymes such as cytochrome P450 are involved in the metabolism of Linezolid, affecting its metabolic flux and metabolite levels . Linezolid’s interaction with these enzymes can influence its pharmacokinetics and pharmacodynamics .
Transport and Distribution
Linezolid is transported and distributed within cells and tissues through passive diffusion and active transport mechanisms . It interacts with transporters and binding proteins that facilitate its uptake and distribution . Linezolid’s localization and accumulation within cells can affect its therapeutic efficacy and toxicity .
Subcellular Localization
Linezolid’s subcellular localization is primarily within the cytoplasm, where it interacts with ribosomes . It can also localize to mitochondria, affecting mitochondrial protein synthesis and function . Targeting signals and post-translational modifications may direct Linezolid to specific cellular compartments, influencing its activity and function .
準備方法
Synthetic Routes and Reaction Conditions: Linezolid is synthesized through a multi-step process involving several key intermediates. One common method involves the preparation of ®-N-[[3-[3-fluoro-4-morpholinyl]phenyl]-2-oxo-5-oxazolidinyl]methanol, which is then converted to Linezolid through a series of reactions . The process typically includes steps such as substitution, cyclization, and amidation .
Industrial Production Methods: Industrial production of Linezolid involves optimizing the synthetic route to ensure high yield and purity. The process is designed to be cost-effective and environmentally friendly, with minimal waste and by-products . Key steps include the use of metal bases and specific reaction conditions to achieve the desired product .
化学反応の分析
Types of Reactions: Linezolid undergoes various chemical reactions, including oxidation, reduction, and substitution . These reactions are essential for its synthesis and modification.
Common Reagents and Conditions: Common reagents used in the synthesis of Linezolid include potassium phthalimide, metal bases, and specific solvents . Reaction conditions such as temperature, pressure, and pH are carefully controlled to ensure optimal results.
Major Products Formed: The major product formed from these reactions is Linezolid itself, along with intermediates such as ®-N-[[3-[3-fluoro-4-morpholinyl]phenyl]-2-oxo-5-oxazolidinyl]methanol .
類似化合物との比較
Tedizolid: Another oxazolidinone antibiotic with a similar mechanism of action but different pharmacokinetic properties.
Vancomycin: A glycopeptide antibiotic used to treat Gram-positive infections, but with a different mechanism of action.
Uniqueness of Linezolid: Linezolid is unique due to its ability to inhibit the initiation of protein synthesis, a mechanism not commonly found in other antibiotics . Its effectiveness against resistant bacteria and its relatively low resistance rates make it a valuable option in the treatment of serious infections .
特性
IUPAC Name |
N-[[(5S)-3-(3-fluoro-4-morpholin-4-ylphenyl)-2-oxo-1,3-oxazolidin-5-yl]methyl]acetamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C16H20FN3O4/c1-11(21)18-9-13-10-20(16(22)24-13)12-2-3-15(14(17)8-12)19-4-6-23-7-5-19/h2-3,8,13H,4-7,9-10H2,1H3,(H,18,21)/t13-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
TYZROVQLWOKYKF-ZDUSSCGKSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(=O)NCC1CN(C(=O)O1)C2=CC(=C(C=C2)N3CCOCC3)F | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC(=O)NC[C@H]1CN(C(=O)O1)C2=CC(=C(C=C2)N3CCOCC3)F | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C16H20FN3O4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID5046489 | |
| Record name | Linezolid | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5046489 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
337.35 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Linezolid | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014739 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
1.44e+00 g/L | |
| Record name | Linezolid | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00601 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Linezolid | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014739 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Linezolid exerts its antibacterial effects by interfering with bacterial protein translation. It binds to a site on the bacterial 23S ribosomal RNA of the 50S subunit and prevents the formation of a functional 70S initiation complex, which is essential for bacterial reproduction, thereby preventing bacteria from dividing. Point mutations in the bacterial 23S rRNA can lead to linezolid resistance, and the development of linezolid-resistant _Enterococcus faecium_ and _Staphylococcus aureus_ have been documented during its clinical use. As antimicrobial susceptibility patterns are geographically distinct, local antibiograms should be consulted to ensure adequate coverage of relevant pathogens prior to use., Linezolid is a synthetic oxazolidinone anti-infective agent that is structurally unrelated to other anti-infectives commercially available in the US. In contrast to other anti-infectives that inhibit bacterial protein synthesis, linezolid acts early in translation by binding to a site on the bacterial 23S ribosomal RNA of the 50S subunit and preventing the formation of a functional 70S initiation complex, which is an essential component of the bacterial translation process., Linezolid acts via inhibition of protein synthesis. It bind to a site on the bacterial 23S ribosomal RNA of the 50S subunit and prevents the formation of a functional 70S initiation complex. This step is essential for the bacterial translation process., Linezolid is an oxazolidinone antibiotic that is increasingly used to treat drug-resistant, gram-positive pathogens. The mechanism of action is inhibition of bacterial protein synthesis. Optic and/or peripheral neuropathy and lactic acidosis are reported side effects, but the underlying pathophysiological mechanism has not been unravelled. Mitochondrial ultrastructure, mitochondrial respiratory chain enzyme activity /were studied/, and mitochondrial DNA (mtDNA) in muscle, liver, and kidney samples obtained from a patient who developed optic neuropathy, encephalopathy, skeletal myopathy, lactic acidosis, and renal failure after prolonged use of linezolid. In addition, mtDNA, respiratory chain enzyme activity, and protein amount in muscle and liver samples obtained from experimental animals that received linezolid or placebo /were evaluated/. In the patient, mitochondrial respiratory chain enzyme activity was decreased in affected tissues, without ultrastructural mitochondrial abnormalities and without mutations or depletion of mtDNA. In the experimental animals, linezolid induced a dose- and time-dependent decrease of the activity of respiratory chain complexes containing mtDNA-encoded subunits and a decreased amount of protein of these complexes, whereas the amount of mtDNA was normal. These results provide direct evidence that linezolid inhibits mitochondrial protein synthesis with potentially severe clinical consequences. | |
| Record name | Linezolid | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00601 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | LINEZOLID | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7478 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
White crystals from ethyl acetate and hexanes | |
CAS No. |
165800-03-3 | |
| Record name | Linezolid | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=165800-03-3 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Linezolid [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0165800033 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Linezolid | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00601 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Linezolid | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5046489 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | LINEZOLID | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/ISQ9I6J12J | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | LINEZOLID | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7478 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Linezolid | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014739 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
181.5-182.5 °C | |
| Record name | Linezolid | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00601 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | LINEZOLID | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7478 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Synthesis routes and methods
Procedure details






Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the mechanism of action of Linezolid?
A1: Linezolid exerts its antibacterial effect by binding to the bacterial ribosome, specifically at the peptidyl transferase center (PTC) located within the 50S ribosomal subunit []. This binding interferes with the formation of the 70S initiation complex, which is essential for protein synthesis [, ].
Q2: What is the downstream effect of Linezolid binding to the ribosome?
A2: By preventing the formation of the 70S initiation complex, Linezolid effectively inhibits the initiation phase of bacterial protein synthesis [, ]. This bacteriostatic action prevents bacterial growth and allows the host's immune system to clear the infection.
Q3: What is the molecular formula and weight of Linezolid?
A3: The molecular formula of Linezolid is C16H20FN3O4, and its molecular weight is 337.34 g/mol.
Q4: Is there any spectroscopic data available for Linezolid?
A4: Yes, UV-Spectrophotometric methods have been developed and validated for the quantification of Linezolid in bulk material and tablet formulations []. These methods utilize the absorbance of Linezolid in the UV-visible range, with a maximum absorbance observed at 251 nm [].
Q5: Is Linezolid compatible with commonly used intravenous fluids?
A5: Yes, studies have demonstrated that Linezolid is stable in commonly used intravenous fluids like sodium lactate, 0.9% sodium chloride, and 5% and 10% glucose solutions for up to 34 days at 25°C [].
Q6: How is Linezolid eliminated from the body?
A6: Approximately 50% of Linezolid is eliminated through renal clearance, indicating the importance of renal function in its pharmacokinetics []. The remaining portion is likely metabolized, with further research needed to fully elucidate the metabolic pathways.
Q7: What is the relationship between Linezolid exposure and thrombocytopenia?
A7: Studies suggest that thrombocytopenia, a decrease in platelet count, is primarily driven by Linezolid's inhibition of platelet formation (myelosuppression) rather than enhanced platelet destruction []. Higher Linezolid concentrations (Cmin > 2 mg/L and AUC0-24 > 160 mg*h/L) have been associated with an increased risk of cytopenias, including thrombocytopenia, in patients treated for multidrug-resistant tuberculosis [].
Q8: Does impaired renal function influence Linezolid pharmacokinetics and the risk of thrombocytopenia?
A8: Yes, impaired renal function can lead to the accumulation of both Linezolid and its metabolite PNU142300, resulting in higher drug exposure [, ]. This accumulation increases the risk of thrombocytopenia in patients with renal insufficiency [, , ]. Dose adjustments might be necessary for these patients to minimize the risk of adverse effects.
Q9: Does co-administration of rifampin impact Linezolid exposure?
A9: Yes, rifampin can significantly reduce Linezolid exposure through a drug-drug interaction [, ]. Rifampin is a potent inducer of P-glycoproteins, which are efflux transporters that can actively remove drugs from cells []. It is believed that rifampin induces the expression of these pumps, leading to increased efflux and decreased plasma levels of Linezolid []. This interaction highlights the importance of therapeutic drug monitoring and potential dose adjustments when combining these antibiotics.
Q10: What is the in vitro activity of Linezolid against different Staphylococcus aureus strains?
A10: Linezolid demonstrates excellent in vitro activity against various Staphylococcus aureus strains, including methicillin-resistant S. aureus (MRSA) [, , , , , ]. It exhibits MIC values ranging from 0.5 to 4 µg/mL against both methicillin-resistant and methicillin-susceptible strains [].
Q11: Has Linezolid shown efficacy in treating MRSA infections in animal models?
A11: Yes, studies using a guinea pig model of foreign-body infection have shown that Linezolid, particularly in combination with rifampin, effectively reduces bacterial load and improves cure rates compared to monotherapy [].
Q12: What are the findings from clinical trials comparing Linezolid to vancomycin in treating MRSA infections?
A12: Several studies have compared the effectiveness of Linezolid and vancomycin in treating MRSA infections.
- One large retrospective cohort study involving veterans with MRSA infections found that linezolid therapy was associated with a shorter length of stay compared to vancomycin therapy [].
- Another study focusing on MRSA ventilator-associated pneumonia (VAP) observed a trend toward higher cure rates with Linezolid compared to vancomycin [].
Q13: What are the known mechanisms of resistance to Linezolid?
A13: Resistance to Linezolid can arise through several mechanisms, primarily affecting the drug's binding site on the ribosome [].
Q14: What are the main safety concerns associated with Linezolid use?
A14: While generally well-tolerated, Linezolid has been associated with several adverse effects, with hematological toxicity, particularly thrombocytopenia, being a significant concern [, , , , , ]. The risk of thrombocytopenia appears to be dose- and duration-dependent and is more pronounced in patients with pre-existing renal impairment or those receiving prolonged treatment [, , , ].
Q15: Are there any specific patient populations that require closer monitoring for Linezolid-induced thrombocytopenia?
A15: Yes, patients with pre-existing renal insufficiency, low baseline platelet counts, and those receiving prolonged Linezolid therapy require close monitoring for thrombocytopenia [, , ]. Additionally, patients with bacteremia and low baseline hemoglobin levels may also be at increased risk [].
Q16: Does Linezolid effectively penetrate the central nervous system?
A16: Yes, Linezolid demonstrates good penetration into the cerebrospinal fluid (CSF) []. Studies in neurosurgical patients have shown that Linezolid achieves adequate concentrations in the CSF, supporting its use in treating central nervous system infections caused by susceptible Gram-positive pathogens [].
Q17: What analytical methods are commonly employed to quantify Linezolid concentrations?
A17: High-performance liquid chromatography (HPLC) is a widely used technique for quantifying Linezolid concentrations in various biological matrices, including serum and CSF []. Additionally, UV-Spectrophotometric methods have been developed and validated for determining Linezolid content in bulk materials and tablet formulations [].
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。
![N-methyl-N-[3-(3-methyl[1,2,4]triazolo[4,3-b]pyridazin-6-yl)phenyl]acetamide](/img/structure/B1675408.png)