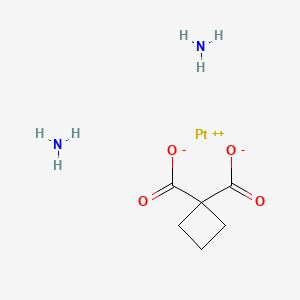
カルボプラチン
概要
説明
カルボプラチンは、白金系抗がん剤であり、卵巣がん、肺がん、頭頸部がん、脳腫瘍、神経芽細胞腫など、さまざまな種類のがんの治療に使用されます . シスプラチンよりも毒性が低い類似体として開発され、1989年に医療用として承認されました . カルボプラチンは、DNAの複製を阻害することでがん細胞の増殖を抑制します .
2. 製法
合成経路と反応条件: カルボプラチンは、シスプラチンとシクロブタン-1,1-ジカルボン酸をアンモニア存在下で反応させることで合成されます . 反応条件は、通常、カルボプラチン錯体の生成を促進するために混合物を加熱する必要があります。
工業生産方法: 工業的には、カルボプラチンは、シスプラチンを水に溶解し、シクロブタン-1,1-ジカルボン酸とアンモニアを加えることで製造されます。 次に、混合物を加熱して反応を促進し、生成されたカルボプラチンを結晶化によって精製します .
作用機序
カルボプラチンは、細胞内のDNA分子に鎖間および鎖内架橋を引き起こす反応性白金錯体を形成することで効果を発揮します . このDNA構造の修飾は、DNA合成を阻害し、細胞周期に影響を与え、最終的に細胞死をもたらします . カルボプラチンの分子標的はDNAであり、カルボプラチンはDNAに共有結合し、複製プロセスを妨害します .
類似の化合物:
シスプラチン: カルボプラチンの前身であり、毒性は高いが作用機序は類似しています.
オキサリプラチン: 別の白金系抗がん剤であり、活性スペクトルと毒性プロファイルが異なります.
比較:
シスプラチン vs. カルボプラチン: カルボプラチンは、特に腎毒性と消化器系の副作用に関して、シスプラチンよりも毒性が低いです.
オキサリプラチン vs. カルボプラチン: オキサリプラチンは、活性スペクトルが異なり、しばしば大腸がんの治療に使用されます。
カルボプラチンは、シスプラチンよりも毒性が低く、効果が同等であるため、特にシスプラチンの副作用に耐えられない患者にとって、化学療法レジメンにおいてシスプラチンに代わる貴重な選択肢となっています .
科学的研究の応用
Carboplatin has a wide range of scientific research applications:
生化学分析
Biochemical Properties
Carboplatin predominantly acts by attaching alkyl groups to the nucleotides, leading to the formation of monoadducts, and DNA fragmenting when repair enzymes attempt to correct the error . The copper transporter 1 seems to be of particular importance for carboplatin uptake in tumor cells .
Cellular Effects
Carboplatin has a profound impact on various types of cells and cellular processes. It can cause a decrease in white blood cells (neutropenia) which can cause complications . Common side effects include low blood cell levels, nausea, and electrolyte problems .
Molecular Mechanism
Carboplatin works by interfering with the duplication of DNA . It binds covalently to DNA and reacts with intrastrand and interstrand DNA sites to inhibit the production of proteins, while also inhibiting transcription .
Temporal Effects in Laboratory Settings
Carboplatin has a myelosuppressive effect, causing the blood cell and platelet output of bone marrow in the body to decrease quite dramatically, sometimes as low as 10% of its usual production levels . The nadir of this myelosuppression usually occurs 21–28 days after the first treatment .
Dosage Effects in Animal Models
In animal models, a range of physiological and morphological effects was produced using doses calculated from the recommended therapeutic range (200–400 mg/m2) . A carboplatin dose reduction of as little as 10% was suggested to result in a doubling of 5-year relapse rate .
Metabolic Pathways
Carboplatin is involved in metabolic pathways that lead to DNA damage. In the presence of glutathione S-transferase (GST), GSH combines with carboplatin to form a Pt-GSH complex that is excreted from cells by multidrug resistance-associated proteins (MRPs), consequently reducing intracellular carboplatin concentration .
Transport and Distribution
Carboplatin is transported and distributed within cells and tissues through various transporters. The copper transporter 1 seems to be of particular importance for carboplatin uptake in tumor cells . Carboplatin showed an obviously different pharmacokinetic characteristic from cisplatin and oxaliplatin. On the one hand, carboplatin has the highest proportion of Pt distribution in ultrafiltrate plasma .
Subcellular Localization
The distribution of total platinum in the cytosol is higher than in the mitochondria, followed by the nucleus . Enrichment of platinum in mitochondria may affect the respiratory chain or energy metabolism, and further lead to cell apoptosis .
準備方法
Synthetic Routes and Reaction Conditions: Carboplatin is synthesized through the reaction of cisplatin with cyclobutane-1,1-dicarboxylic acid in the presence of ammonia . The reaction conditions typically involve heating the mixture to facilitate the formation of the carboplatin complex.
Industrial Production Methods: In industrial settings, carboplatin is produced by dissolving cisplatin in water and adding cyclobutane-1,1-dicarboxylic acid and ammonia. The mixture is then heated to promote the reaction, and the resulting carboplatin is purified through crystallization .
化学反応の分析
反応の種類: カルボプラチンは、置換反応など、さまざまな化学反応を起こします。 注目すべき反応の1つは、塩化物イオンによる活性化であり、これはマロネート部分の除去とシスプラチンの生成をもたらします .
一般的な試薬と条件:
塩化物イオン: カルボプラチンを活性化してシスプラチンに変換するために使用されます.
酸性条件: 強酸性環境は、カルボプラチンをシスプラチンに変換します.
主要な生成物:
4. 科学研究への応用
カルボプラチンは、さまざまな科学研究に幅広く応用されています:
類似化合物との比較
Cisplatin: The predecessor of carboplatin, known for its higher toxicity but similar mechanism of action.
Oxaliplatin: Another platinum-based chemotherapy drug with a different spectrum of activity and toxicity profile.
Comparison:
Cisplatin vs. Carboplatin: Carboplatin is less toxic than cisplatin, particularly in terms of nephrotoxicity and gastrointestinal side effects.
Oxaliplatin vs. Carboplatin: Oxaliplatin has a different spectrum of activity and is often used to treat colorectal cancer.
Carboplatin’s reduced toxicity and similar efficacy make it a valuable alternative to cisplatin in chemotherapy regimens, particularly for patients who cannot tolerate the side effects of cisplatin .
特性
IUPAC Name |
azanide;cyclobutane-1,1-dicarboxylic acid;platinum(2+) | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C6H8O4.2H2N.Pt/c7-4(8)6(5(9)10)2-1-3-6;;;/h1-3H2,(H,7,8)(H,9,10);2*1H2;/q;2*-1;+2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
VSRXQHXAPYXROS-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1CC(C1)(C(=O)O)C(=O)O.[NH2-].[NH2-].[Pt+2] | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C6H12N2O4Pt | |
| Record name | Carboplatin | |
| Source | Wikipedia | |
| URL | https://en.wikipedia.org/wiki/Carboplatin | |
| Description | Chemical information link to Wikipedia. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Weight |
371.25 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
Sol in water, Water > 15 (mg/mL), pH 4 Acetate Buffer 5 - 10 (mg/mL), pH 9 Carbonate Buffer 5 - 10 (mg/mL), 10% Ethanol/H2O 5 - 10 (mg/mL), 95% Ethanol/H < 1 (mg/mL), 0.1NHC1 5 -10 (mg/mL), 0.1NNaOH 5 -10 (mg/mL), Methanol < 1 (mg/mL), Chloroform < 5 (mg/mL), Dimethylsulfoxide 5 (mg/mL), Acetic Acid < 1 (mg/mL), Trifluoroacetic Acid < 1 (mg/mL) | |
| Record name | CARBOPLATIN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6957 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | CARBOPLATIN | |
| Source | NCI Investigational Drugs | |
| URL | http://dtp.nci.nih.gov/NCI-InvestigationalDrugsCI92/241240%20(1992).txt | |
| Description | An investigational drug is one that is under study but does not have permission from the U.S. Food and Drug Administration (FDA) to be legally marketed and sold in the United States. NCI provides the investigational drug to the physicians who are participating in clinical trials or TRC protocols. For more information please visit NCI investigational drug website: https://www.cancer.gov/about-cancer/treatment/drugs/investigational-drug-access-fact-sheet | |
Color/Form |
White crystals | |
CAS No. |
41575-94-4 | |
| Record name | carboplatin | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=758182 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | carboplatin | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=241240 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | carboplatin | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=201345 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Diammine[cyclobutane-1,1-dicarboxylato(2-)-O,O']platinum | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.050.388 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | CARBOPLATIN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6957 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
A: Carboplatin, similar to cisplatin, exerts its cytotoxic effect by binding to DNA. [, , ] This binding primarily results in the formation of platinum-DNA adducts, specifically monoadducts and intra- and interstrand diadducts. [] These adducts interfere with DNA replication and transcription, ultimately triggering cell cycle arrest and apoptosis. [, ]
A: Yes, research using accelerator mass spectrometry has demonstrated that carboplatin forms DNA monoadducts at a rate approximately 100-fold slower than cisplatin. [] This difference in the kinetics of DNA adduct formation may contribute to the lower toxicity profile of carboplatin compared to cisplatin. []
A: Studies indicate that carboplatin can enhance the production and persistence of radiation-induced DNA single-strand breaks (SSBs). [] This effect was observed both when carboplatin was administered before irradiation under hypoxic conditions and when added after irradiation during the post-irradiation recovery period in air. [] This suggests a synergistic interaction between carboplatin and radiation therapy.
A: Research on human laryngeal squamous carcinoma cells (HN-3) showed that carboplatin treatment led to down-regulation of epidermal growth factor receptor (EGFR) expression. [] This effect was associated with increased reactive oxygen species (ROS) generation within the cells, indicating a potential mechanism for carboplatin's antitumor activity. []
A: Yes, computational studies have investigated the interaction between carboplatin and a B12N12 nano-cluster. [] This study utilized density functional theory (DFT) calculations with the B3P86 functional to explore the binding interactions and energy differences between various isomers of the carboplatin-nano-cluster complex. []
A: A study on a patient undergoing hemodialysis revealed a significantly lower area under the curve (AUC) of free platinum when carboplatin was administered shortly before hemodialysis. [] This suggests a substantial removal of carboplatin from the systemic circulation during hemodialysis, highlighting the importance of timing chemotherapy administration in this patient population.
A: In vitro experiments utilizing a bovine brain capillary endothelial cell model showed that hyperbaric oxygenation increased the transendothelial permeability of carboplatin. [] This suggests a potential strategy for enhancing carboplatin delivery to the brain, which is particularly relevant for treating brain tumors.
A: Several studies employed various cancer cell lines to investigate the in vitro efficacy of carboplatin, including human laryngeal squamous carcinoma cells (HN-3), [] three glioma cell lines (U251MG, SNB19, and LNZ308), [] two adenocarcinoma cell lines (MiaPaCa and SW480), [] five canine transitional cell carcinoma (TCC) cell lines, [] T24 human bladder cancer cells, [] COR-L23/P human non-small-cell lung carcinoma (NSCLC) cells, [] and two triple-negative breast cancer cell lines (CF41.Mg and MDA-MB-468). []
ANone: Several preclinical studies explored carboplatin-based combination therapies:
- Carboplatin and Difluoromethylornithine (DFMO): Synergistic or additive effects were observed in a majority of the tested glioma and adenocarcinoma cell lines. []
- Carboplatin and Vorinostat (SAHA): Synergy was noted in one glioma cell line and in the MiaPaCa cell line with short exposure times. []
- Carboplatin and Docetaxel: Synergistic effects were observed in two out of three glioma cell lines and in the SW480 cell line with short exposures. []
- Carboplatin and Gemcitabine: Synergistic activity at biologically achievable concentrations was observed in three out of five canine TCC cell lines. []
- Carboplatin and XR5944: Enhanced anti-tumor activity was observed in COR-L23/P human non-small-cell lung carcinoma (NSCLC) xenograft models when XR5944 was administered before carboplatin. []
ANone: Numerous clinical trials have investigated the efficacy and safety of carboplatin in different cancer types:
- Head and Neck Cancer: A retrospective cohort study on US veterans with head and neck squamous cell carcinoma (HNSCC) suggested that carboplatin-based chemoradiotherapy was associated with improved overall survival compared to cetuximab-based chemoradiotherapy. [] This difference was particularly pronounced in patients with oropharynx cancer.
- A phase III randomized trial found that docetaxel-carboplatin was comparable to paclitaxel-carboplatin in terms of progression-free survival and response rate as a first-line chemotherapy regimen. [] Importantly, docetaxel-carboplatin was associated with significantly less neurotoxicity.
- A phase II trial explored the combination of gemcitabine/carboplatin with or without trastuzumab as first-line treatment for metastatic breast cancer. [] The regimen demonstrated promising activity, particularly in HER2-positive disease.
- A retrospective study from Japan evaluated high-dose ifosfamide, carboplatin, and etoposide combined with peripheral blood stem cell transplantation for male germ cell tumors. [] The treatment was found to be feasible and effective with manageable toxicity.
- A phase II trial evaluated the combination of vinorelbine and gemcitabine as first-line therapy for advanced NSCLC. [] The regimen showed promising activity and tolerability, comparable to standard platinum-based combinations.
- A phase II study investigated a triplet chemotherapy regimen of carboplatin, paclitaxel, and gemcitabine with amifostine support in advanced NSCLC. [] The combination demonstrated promising activity and good tolerability.
- A phase III trial compared nab-paclitaxel plus carboplatin to solvent-based paclitaxel plus carboplatin as first-line treatment for advanced NSCLC. [] Nab-paclitaxel plus carboplatin demonstrated a significantly improved overall response rate, particularly in patients with squamous cell histology.
- A phase II study assessed the feasibility, safety, and efficacy of carboplatin plus S-1 followed by maintenance S-1 therapy for patients with completely resected stage II/IIIA NSCLC. [] The study found that this regimen was not well-tolerated, highlighting the challenges of incorporating carboplatin into adjuvant settings for NSCLC.
- Glioblastoma: A phase 1/2 clinical trial investigated the feasibility and safety of blood-brain barrier (BBB) disruption using an implantable ultrasound system (SonoCloud-9) in combination with intravenous carboplatin in patients with recurrent glioblastoma. [] The study demonstrated the safety and feasibility of this approach, as well as a substantial increase in carboplatin concentration in the peritumoral brain region.
A: While considered less toxic than cisplatin, carboplatin can still cause side effects. Myelosuppression, particularly thrombocytopenia and neutropenia, is a common side effect of carboplatin treatment. [, , , , ] Other reported side effects include nausea, [] diarrhea, [] hepatotoxicity, [] nephrotoxicity, [] neurotoxicity, [] alopecia, [, , ] and hypersensitivity reactions. [, ] The severity and frequency of side effects can vary depending on the dose, schedule, and combination with other drugs.
A: One study explored the use of hyperbaric oxygenation as a method to enhance the transport of carboplatin across the blood-brain barrier. [] This approach showed promising results in vitro and warrants further investigation in clinical settings.
A: Research has explored the use of folate-targeted PEG as a potential carrier for carboplatin analogs. [] While the folate-targeted conjugates showed efficient cellular uptake via the folate receptor-mediated endocytosis pathway, they formed relatively few DNA adducts and exhibited higher IC50 values compared to carboplatin and non-targeted analogs. [] Further research is needed to optimize the design and delivery of targeted carboplatin conjugates.
ANone: Various analytical techniques have been used to investigate carboplatin, including:
- Accelerator Mass Spectrometry (AMS): Used to measure the kinetics of carboplatin-DNA adduct formation and drug uptake in cells. []
- Fluorescence-Based Microplate Cell Proliferation Assay: Employed to assess the effects of carboplatin on cell proliferation in vitro. []
- Propidium Iodide Staining: Used to evaluate cell cycle distribution in response to carboplatin treatment. []
- Caspase 3 and 7 Enzymatic Activity Measurement: Employed to assess apoptosis induction by carboplatin. []
- Atom Absorbing Spectrometry: Used to measure carboplatin concentration in axillary lymph nodes after regional injection of carboplatin-activated carbon suspension. []
- Immunofluorescence, Western Blotting, and qRT-PCR: Used to assess the expression levels of specific proteins and genes in response to carboplatin treatment. []
- Magnetic Resonance Imaging (MRI): Used to monitor tumor size and drug distribution in preclinical and clinical settings. [, ]
A: Research using a bovine brain capillary endothelial cell model suggests that carboplatin may be a substrate for P-glycoprotein (P-gp), a drug efflux transporter. [] The study demonstrated that verapamil, a P-gp inhibitor, enhanced the transendothelial permeability of carboplatin, similar to its effects on doxorubicin, a known P-gp substrate. [] This finding highlights the potential for drug-transporter interactions to influence carboplatin distribution and efficacy.
A: Yes, cisplatin is another platinum-based chemotherapeutic agent often used in cancer treatment. [, , ] While both drugs share a similar mechanism of action, they exhibit different pharmacokinetic and toxicity profiles. Clinical trials have directly compared carboplatin to cisplatin in various cancer types, such as NSCLC and ovarian cancer, with varying results. [, , ] The choice between carboplatin and cisplatin often depends on factors like tumor type, patient characteristics, and potential side effects.
A: Yes, research is ongoing to identify effective non-platinum-based chemotherapy options for various cancers. For instance, one study explored the combination of vinorelbine and gemcitabine as a potential alternative to platinum-containing regimens in advanced NSCLC. [] This combination demonstrated promising activity and a favorable toxicity profile compared to some platinum-based therapies.
ANone: The research on carboplatin exemplifies the collaborative nature of scientific advancements:
- Medicinal Chemistry: Plays a crucial role in designing and synthesizing carboplatin analogs with improved efficacy, pharmacokinetic properties, and reduced toxicity. []
- Pharmacology and Pharmaceutics: Focus on understanding the absorption, distribution, metabolism, and excretion (ADME) of carboplatin, as well as developing optimal drug delivery systems. [, ]
- Oncology and Clinical Research: Drive the evaluation of carboplatin in clinical trials, investigating its efficacy, safety, and optimal use in different cancer types and patient populations. [, , , , , , , , , , , , ]
- Molecular Biology and Genetics: Contribute to understanding the mechanisms of action, resistance, and potential biomarkers for carboplatin therapy. [, , , ]
- Analytical Chemistry: Develops and refines methods for characterizing carboplatin and monitoring its levels in biological samples. [, , ]
- Biomedical Engineering: Develops novel technologies for drug delivery, such as the implantable ultrasound system used to enhance carboplatin delivery to the brain. []
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。


![2-[[4-[(2-Amino-3-sulfanylpropyl)amino]-2-naphthalen-1-ylbenzoyl]amino]-4-methylpentanoic acid](/img/structure/B1684560.png)