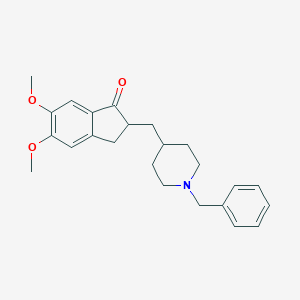
Donepezil
カタログ番号 B133215
分子量: 379.5 g/mol
InChIキー: ADEBPBSSDYVVLD-UHFFFAOYSA-N
注意: 研究専用です。人間または獣医用ではありません。
Patent
US08124783B2
Procedure details


There are many processes as disclosed in the prior arts for producing donepezil of formula 1. U.S. Pat. No. 4,895,841 wherein substituted 1-indanone-2-phosphonate prepared from 2-bromo-5,6-dimethoxyindanone and triethyl phosphite, is treated with 1-benzylpiperidine-4-carboxaldehyde in the presence of a strong base, such as lithium diisopropylamide (LDA), followed by catalytic reduction using palladium on carbon in tetrahydrofuran (40 volumes) to yield donepezil with an overall yield of 50.8%. This process however suffers with few limitations i.e. it employs triphenylphosphonium methoxymethyl chloride, which is expensive and toxic and the overall yield of this process is quite low. (scheme 1).
[Compound]
Name
substituted 1-indanone-2-phosphonate
Quantity
0 (± 1) mol
Type
reactant
Reaction Step One







Name
Yield
50.8%
Identifiers


|
REACTION_CXSMILES
|
Br[CH:2]1[CH2:10][C:9]2[C:4](=[CH:5][C:6]([O:13][CH3:14])=[C:7]([O:11][CH3:12])[CH:8]=2)[C:3]1=[O:15].P(OCC)(OCC)OCC.[CH2:26]([N:33]1[CH2:38][CH2:37][CH:36]([CH:39]=O)[CH2:35][CH2:34]1)[C:27]1[CH:32]=[CH:31][CH:30]=[CH:29][CH:28]=1.C([N-]C(C)C)(C)C.[Li+]>[Pd].O1CCCC1>[CH3:12][O:11][C:7]1[CH:8]=[C:9]2[CH2:10][CH:2]([CH2:39][CH:36]3[CH2:35][CH2:34][N:33]([CH2:26][C:27]4[CH:28]=[CH:29][CH:30]=[CH:31][CH:32]=4)[CH2:38][CH2:37]3)[C:3](=[O:15])[C:4]2=[CH:5][C:6]=1[O:13][CH3:14] |f:3.4|
|
Inputs


Step One
[Compound]
|
Name
|
substituted 1-indanone-2-phosphonate
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
|
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
BrC1C(C2=CC(=C(C=C2C1)OC)OC)=O
|
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
P(OCC)(OCC)OCC
|
Step Two
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C1=CC=CC=C1)N1CCC(CC1)C=O
|
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C)(C)[N-]C(C)C.[Li+]
|
Step Three
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
catalyst
|
|
Smiles
|
[Pd]
|
Step Four
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
solvent
|
|
Smiles
|
O1CCCC1
|
Conditions


Other
|
Conditions are dynamic
|
1
|
|
Details
|
See reaction.notes.procedure_details.
|
Outcomes


Product
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
COC=1C=C2C(=CC1OC)C(=O)C(C2)CC3CCN(CC3)CC=4C=CC=CC4
|
Measurements
| Type | Value | Analysis |
|---|---|---|
| YIELD: PERCENTYIELD | 50.8% |
Source


|
Source
|
Open Reaction Database (ORD) |
|
Description
|
The Open Reaction Database (ORD) is an open-access schema and infrastructure for structuring and sharing organic reaction data, including a centralized data repository. The ORD schema supports conventional and emerging technologies, from benchtop reactions to automated high-throughput experiments and flow chemistry. Our vision is that a consistent data representation and infrastructure to support data sharing will enable downstream applications that will greatly improve the state of the art with respect to computer-aided synthesis planning, reaction prediction, and other predictive chemistry tasks. |
Patent
US08124783B2
Procedure details


There are many processes as disclosed in the prior arts for producing donepezil of formula 1. U.S. Pat. No. 4,895,841 wherein substituted 1-indanone-2-phosphonate prepared from 2-bromo-5,6-dimethoxyindanone and triethyl phosphite, is treated with 1-benzylpiperidine-4-carboxaldehyde in the presence of a strong base, such as lithium diisopropylamide (LDA), followed by catalytic reduction using palladium on carbon in tetrahydrofuran (40 volumes) to yield donepezil with an overall yield of 50.8%. This process however suffers with few limitations i.e. it employs triphenylphosphonium methoxymethyl chloride, which is expensive and toxic and the overall yield of this process is quite low. (scheme 1).
[Compound]
Name
substituted 1-indanone-2-phosphonate
Quantity
0 (± 1) mol
Type
reactant
Reaction Step One







Name
Yield
50.8%
Identifiers


|
REACTION_CXSMILES
|
Br[CH:2]1[CH2:10][C:9]2[C:4](=[CH:5][C:6]([O:13][CH3:14])=[C:7]([O:11][CH3:12])[CH:8]=2)[C:3]1=[O:15].P(OCC)(OCC)OCC.[CH2:26]([N:33]1[CH2:38][CH2:37][CH:36]([CH:39]=O)[CH2:35][CH2:34]1)[C:27]1[CH:32]=[CH:31][CH:30]=[CH:29][CH:28]=1.C([N-]C(C)C)(C)C.[Li+]>[Pd].O1CCCC1>[CH3:12][O:11][C:7]1[CH:8]=[C:9]2[CH2:10][CH:2]([CH2:39][CH:36]3[CH2:35][CH2:34][N:33]([CH2:26][C:27]4[CH:28]=[CH:29][CH:30]=[CH:31][CH:32]=4)[CH2:38][CH2:37]3)[C:3](=[O:15])[C:4]2=[CH:5][C:6]=1[O:13][CH3:14] |f:3.4|
|
Inputs


Step One
[Compound]
|
Name
|
substituted 1-indanone-2-phosphonate
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
|
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
BrC1C(C2=CC(=C(C=C2C1)OC)OC)=O
|
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
P(OCC)(OCC)OCC
|
Step Two
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C1=CC=CC=C1)N1CCC(CC1)C=O
|
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C)(C)[N-]C(C)C.[Li+]
|
Step Three
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
catalyst
|
|
Smiles
|
[Pd]
|
Step Four
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
solvent
|
|
Smiles
|
O1CCCC1
|
Conditions


Other
|
Conditions are dynamic
|
1
|
|
Details
|
See reaction.notes.procedure_details.
|
Outcomes


Product
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
COC=1C=C2C(=CC1OC)C(=O)C(C2)CC3CCN(CC3)CC=4C=CC=CC4
|
Measurements
| Type | Value | Analysis |
|---|---|---|
| YIELD: PERCENTYIELD | 50.8% |
Source


|
Source
|
Open Reaction Database (ORD) |
|
Description
|
The Open Reaction Database (ORD) is an open-access schema and infrastructure for structuring and sharing organic reaction data, including a centralized data repository. The ORD schema supports conventional and emerging technologies, from benchtop reactions to automated high-throughput experiments and flow chemistry. Our vision is that a consistent data representation and infrastructure to support data sharing will enable downstream applications that will greatly improve the state of the art with respect to computer-aided synthesis planning, reaction prediction, and other predictive chemistry tasks. |
