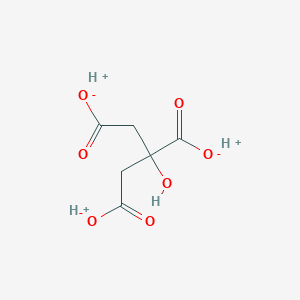
Citric acid
Übersicht
Beschreibung
Citric acid is a weak organic acid with the molecular formula C₆H₈O₇. It is a naturally occurring compound found in citrus fruits such as lemons, limes, and oranges. This compound is a key intermediate in the this compound cycle, which is essential for the metabolism of all aerobic organisms. It is widely used in the food and beverage industry as a flavoring and preservative agent, and it also has applications in pharmaceuticals, cosmetics, and cleaning products .
Wirkmechanismus
Target of Action
Citric acid, a key intermediate in metabolism, primarily targets metabolic processes in the body . It is an acid compound found in citrus fruits . The salts of this compound, known as citrates, can be used as anticoagulants due to their calcium-chelating ability . This compound is also one of the active ingredients in Phexxi, a non-hormonal contraceptive agent .
Mode of Action
The mode of action of this compound involves disrupting the cellular membrane of microorganisms, leading to leakage of cellular contents and ultimately cell death . Additionally, this compound can lower the pH of the food matrix, creating an unfavorable environment for microbial growth . Citrate, a weak base, reacts with hydrochloric acid in the stomach to raise the pH . It is further metabolized to bicarbonate, which then acts as a systemic alkalizing agent, raising the pH of the blood and urine .
Biochemical Pathways
This compound plays a crucial role in the this compound cycle, a central driver of cellular respiration . It takes acetyl CoA, produced by the oxidation of pyruvate and originally derived from glucose, as its starting material . In a series of redox reactions, it harvests much of its bond energy in the form of NADH, FADH2, and ATP molecules . The reduced electron carriers, NADH and FADH2, generated in the this compound cycle will pass their electrons into the electron transport chain and, through oxidative phosphorylation, will generate most of the ATP produced in cellular respiration .
Pharmacokinetics
This compound’s pharmacokinetics properties are influenced by its physicochemical properties . The functionality of this compound is due to its three carboxylic groups and one hydroxyl group . These allow it to be used in many ways, including its ability to be used as a crosslinker to form biodegradable polymers and as a co-former in co-amorphous and co-crystal applications .
Result of Action
The this compound cycle releases two carbon dioxide molecules and produces three NADH, one FADH2, and one ATP or GTP . This set of reactions regenerates the starting molecule, oxaloacetate, so the cycle can repeat . Overall, one turn of the this compound cycle releases two carbon dioxide molecules and produces three NADH, one FADH2, and one ATP or GTP .
Action Environment
This compound acts as a preservative in many processed foods, keeping them fresh by slowing or helping prevent the formation of bacteria, mold, yeast, and fungus . It retains a food’s color, flavor, and texture, increasing its shelf life . At high ionic strength, colloidal particles will flocculate due to a secondary minimum, resulting in aggregates that are dense and easily redispersed .
Biochemische Analyse
Biochemical Properties
Citric acid plays a crucial role in biochemical reactions, particularly in the this compound cycle. It interacts with several enzymes and biomolecules during this cycle. One of the primary enzymes it interacts with is citrate synthase, which catalyzes the condensation of acetyl-CoA and oxaloacetate to form this compound . This compound is then converted to isocitrate by the enzyme aconitase. This conversion involves the intermediate formation of cis-aconitate . Isocitrate is subsequently oxidized by isocitrate dehydrogenase to form alpha-ketoglutarate, releasing carbon dioxide and reducing NAD+ to NADH . These interactions highlight the importance of this compound in energy production and metabolic regulation.
Cellular Effects
This compound influences various cellular processes, including cell signaling pathways, gene expression, and cellular metabolism. In the this compound cycle, this compound is involved in the production of ATP, which is the primary energy currency of the cell . The cycle also generates NADH and FADH₂, which are used in the electron transport chain to produce additional ATP . This compound can also affect gene expression by modulating the activity of transcription factors and enzymes involved in metabolic pathways . Additionally, this compound has been shown to have antioxidant properties, reducing oxidative stress and inflammation in cells .
Molecular Mechanism
At the molecular level, this compound exerts its effects through various binding interactions and enzymatic reactions. In the this compound cycle, this compound binds to and activates citrate synthase, leading to the formation of this compound from acetyl-CoA and oxaloacetate . The enzyme aconitase then catalyzes the isomerization of this compound to isocitrate via cis-aconitate . Isocitrate dehydrogenase further oxidizes isocitrate to alpha-ketoglutarate, producing NADH in the process . These reactions are tightly regulated by feedback mechanisms to ensure efficient energy production and metabolic balance.
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of this compound can change over time due to its stability and degradation. This compound is relatively stable under normal conditions but can degrade under extreme pH or temperature . Long-term studies have shown that this compound can maintain its inhibitory effects on kidney stone formation by binding to calcium ions and preventing crystallization . Additionally, this compound’s antioxidant properties can persist over time, providing sustained protection against oxidative stress .
Dosage Effects in Animal Models
The effects of this compound vary with different dosages in animal models. Studies have shown that this compound is safe for use in animal feed up to a concentration of 30,000 mg/kg . At higher doses, this compound can cause gastrointestinal upset and metabolic alkalosis . In goslings, supplementation with 1.00% this compound improved growth performance, while higher doses led to increased creatinine levels . These findings highlight the importance of dosage in determining the safety and efficacy of this compound in animal models.
Metabolic Pathways
This compound is a central component of the this compound cycle, which is involved in the oxidation of acetyl-CoA to produce ATP, NADH, and FADH₂ . The cycle includes several key enzymes, such as citrate synthase, aconitase, and isocitrate dehydrogenase, which facilitate the conversion of this compound to other intermediates . This compound also interacts with cofactors like NAD+ and FAD, which are essential for the redox reactions in the cycle . These interactions ensure the efficient production of energy and metabolic intermediates.
Transport and Distribution
Within cells, this compound is transported and distributed through various mechanisms. It is produced in the mitochondria during the this compound cycle and can be transported to other cellular compartments as needed . This compound can also form complexes with metal ions, facilitating its transport across cell membranes . Additionally, this compound can be distributed to different tissues through the bloodstream, where it can exert its effects on cellular metabolism and function .
Subcellular Localization
This compound is primarily localized in the mitochondria, where it participates in the this compound cycle . The enzyme citrate synthase, which catalyzes the formation of this compound, is also located in the mitochondrial matrix . This compound can also be found in other subcellular compartments, such as the cytoplasm, where it can influence various metabolic processes . The subcellular localization of this compound is crucial for its role in energy production and metabolic regulation.
Vorbereitungsmethoden
Synthetic Routes and Reaction Conditions: Citric acid can be synthesized through chemical and biological methods. The chemical synthesis involves the use of enzymes to catalyze the conversion of substrates like glycerol into this compound. this method is less common due to its complexity and cost .
Industrial Production Methods: The most common industrial method for producing this compound is through fermentation. This process involves the use of microorganisms such as Aspergillus niger and Candida species. The submerged fermentation technique is widely used, where these microorganisms metabolize carbon sources like molasses and starch-based culture media to produce this compound. Surface fermentation and solid-state fermentation are also employed, using agro-based residues as carbon sources .
Analyse Chemischer Reaktionen
Types of Reactions: Citric acid undergoes various chemical reactions, including:
Oxidation: this compound can be oxidized to form oxaloacetic acid and acetone dicarboxylic acid.
Reduction: It can be reduced to form isothis compound.
Substitution: this compound can react with bases to form citrate salts, such as sodium citrate
Common Reagents and Conditions:
Oxidation: Requires oxidizing agents like potassium permanganate.
Reduction: Involves reducing agents such as hydrogen in the presence of a catalyst.
Substitution: Typically involves bases like sodium hydroxide
Major Products:
Oxidation: Oxaloacetic acid, acetone dicarboxylic acid.
Reduction: Isothis compound.
Substitution: Citrate salts
Wissenschaftliche Forschungsanwendungen
Citric acid has a wide range of applications in scientific research:
Chemistry: Used as a chelating agent to bind metal ions and as a buffer in various chemical reactions.
Biology: Plays a crucial role in the this compound cycle, which is fundamental for cellular respiration and energy production.
Medicine: Used in pharmaceuticals as an anticoagulant, preservative, and flavoring agent. It is also used in the formulation of effervescent tablets and as a component in oral rehydration solutions.
Industry: Employed in the food and beverage industry as a flavoring and preservative agent.
Vergleich Mit ähnlichen Verbindungen
Citric acid belongs to the family of carboxylic acids and shares similarities with other compounds such as:
Acetic Acid: Found in vinegar, it is a simpler carboxylic acid with a single carboxyl group.
Tartaric Acid: Found in grapes, it has two carboxyl groups and is used in baking and winemaking.
Lactic Acid: Produced in muscles during intense exercise, it has a single carboxyl group and is used in food preservation and cosmetics
Uniqueness of this compound:
Multiple Carboxyl Groups: this compound has three carboxyl groups, making it a tricarboxylic acid, which allows it to participate in multiple chemical reactions.
Role in Metabolism: It is a key intermediate in the this compound cycle, essential for energy production in aerobic organisms
This compound’s versatility and wide range of applications make it a unique and valuable compound in various fields.
Eigenschaften
IUPAC Name |
2-hydroxypropane-1,2,3-tricarboxylic acid | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C6H8O7/c7-3(8)1-6(13,5(11)12)2-4(9)10/h13H,1-2H2,(H,7,8)(H,9,10)(H,11,12) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
KRKNYBCHXYNGOX-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C(C(=O)O)C(CC(=O)O)(C(=O)O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C6H8O7, Array | |
| Record name | CITRIC ACID | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/10899 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | CITRIC ACID | |
| Source | ILO-WHO International Chemical Safety Cards (ICSCs) | |
| URL | https://www.ilo.org/dyn/icsc/showcard.display?p_version=2&p_card_id=0855 | |
| Description | The International Chemical Safety Cards (ICSCs) are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. The primary aim of the Cards is to promote the safe use of chemicals in the workplace. | |
| Explanation | Creative Commons CC BY 4.0 | |
| Record name | citric acid | |
| Source | Wikipedia | |
| URL | https://en.wikipedia.org/wiki/Citric_acid | |
| Description | Chemical information link to Wikipedia. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
141633-96-7 | |
| Record name | Citric acid polymer | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=141633-96-7 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
DSSTOX Substance ID |
DTXSID3020332 | |
| Record name | Citric acid | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3020332 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
192.12 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Citric acid appears as colorless, odorless crystals with an acid taste. Denser than water. (USCG, 1999), Dry Powder; Dry Powder, Liquid; Dry Powder, Pellets or Large Crystals; Liquid; Liquid, Other Solid; NKRA; Other Solid; Pellets or Large Crystals; Pellets or Large Crystals, Liquid, Citric acid is a white or colourless, odourless, crystalline solid, having a strongly acid taste. The monohydrate effloresces in dry air, Colorless and odorless crystals , it has an acid taste; [CAMEO] Deliquescent; [CHEMINFO], Solid, COLOURLESS CRYSTALS., White or colourless, odourless, crystalline solid. Monohydrate effloresces in dry air | |
| Record name | CITRIC ACID | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/10899 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | 1,2,3-Propanetricarboxylic acid, 2-hydroxy- | |
| Source | EPA Chemicals under the TSCA | |
| URL | https://www.epa.gov/chemicals-under-tsca | |
| Description | EPA Chemicals under the Toxic Substances Control Act (TSCA) collection contains information on chemicals and their regulations under TSCA, including non-confidential content from the TSCA Chemical Substance Inventory and Chemical Data Reporting. | |
| Record name | CITRIC ACID | |
| Source | EU Food Improvement Agents | |
| URL | https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32012R0231 | |
| Description | Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council Text with EEA relevance | |
| Record name | Citric acid | |
| Source | Haz-Map, Information on Hazardous Chemicals and Occupational Diseases | |
| URL | https://haz-map.com/Agents/2025 | |
| Description | Haz-Map® is an occupational health database designed for health and safety professionals and for consumers seeking information about the adverse effects of workplace exposures to chemical and biological agents. | |
| Explanation | Copyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission. | |
| Record name | Citric acid | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0000094 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
| Record name | CITRIC ACID | |
| Source | ILO-WHO International Chemical Safety Cards (ICSCs) | |
| URL | https://www.ilo.org/dyn/icsc/showcard.display?p_version=2&p_card_id=0855 | |
| Description | The International Chemical Safety Cards (ICSCs) are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. The primary aim of the Cards is to promote the safe use of chemicals in the workplace. | |
| Explanation | Creative Commons CC BY 4.0 | |
| Record name | Citric acid | |
| Source | Joint FAO/WHO Expert Committee on Food Additives (JECFA) | |
| URL | https://www.fao.org/food/food-safety-quality/scientific-advice/jecfa/jecfa-flav/details/en/c/38/ | |
| Description | The flavoring agent databse provides the most recent specifications for flavorings evaluated by JECFA. | |
| Explanation | Permission from WHO is not required for the use of WHO materials issued under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Intergovernmental Organization (CC BY-NC-SA 3.0 IGO) licence. | |
Boiling Point |
Decomposes (NTP, 1992), Decomposes | |
| Record name | CITRIC ACID | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/10899 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | CITRIC ACID | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/911 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Flash Point |
100 °C | |
| Record name | CITRIC ACID | |
| Source | ILO-WHO International Chemical Safety Cards (ICSCs) | |
| URL | https://www.ilo.org/dyn/icsc/showcard.display?p_version=2&p_card_id=0855 | |
| Description | The International Chemical Safety Cards (ICSCs) are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. The primary aim of the Cards is to promote the safe use of chemicals in the workplace. | |
| Explanation | Creative Commons CC BY 4.0 | |
Solubility |
greater than or equal to 100 mg/mL at 72 °F (NTP, 1992), Very soluble in water; freely soluble in ethanol; soluble in ether, In water, 3.83X10+5 mg/L at 25 °C, Solubility in water: 54.0% w/w at 10 °C; 59.2% at 20 °C; 64.3% at 30 °C; 68.6% at 40 °C; 70.9% at 50 °C; 73.5% at 60 °C; 76.2% at 70 °C; 78.8% at 80 °C; 81.4% at 90 °C; 84.0% at 100 °C, Very soluble in ethanol; soluble in ether, ethyl acetate; insoluble in benzene, chloroform, 592.0 mg/mL, Solubility in water, g/100ml at 20 °C: 59, Very soluble in water, slightly soluble in ether, Freely soluble (in ethanol) | |
| Record name | CITRIC ACID | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/10899 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Citric acid | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB04272 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | CITRIC ACID | |
| Source | EU Food Improvement Agents | |
| URL | https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32012R0231 | |
| Description | Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council Text with EEA relevance | |
| Record name | CITRIC ACID | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/911 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Citric acid | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0000094 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
| Record name | CITRIC ACID | |
| Source | ILO-WHO International Chemical Safety Cards (ICSCs) | |
| URL | https://www.ilo.org/dyn/icsc/showcard.display?p_version=2&p_card_id=0855 | |
| Description | The International Chemical Safety Cards (ICSCs) are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. The primary aim of the Cards is to promote the safe use of chemicals in the workplace. | |
| Explanation | Creative Commons CC BY 4.0 | |
| Record name | Citric acid | |
| Source | Joint FAO/WHO Expert Committee on Food Additives (JECFA) | |
| URL | https://www.fao.org/food/food-safety-quality/scientific-advice/jecfa/jecfa-flav/details/en/c/38/ | |
| Description | The flavoring agent databse provides the most recent specifications for flavorings evaluated by JECFA. | |
| Explanation | Permission from WHO is not required for the use of WHO materials issued under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Intergovernmental Organization (CC BY-NC-SA 3.0 IGO) licence. | |
Density |
1.54 at 68 °F (USCG, 1999) - Denser than water; will sink, 1.665 g/cu cm at 20 °C, BULK DENSITY: 56.2 LB/CU FT; HEAT OF SOLN: -3.9 KCAL/MOLE; BUFFERING INDEX: 2.46; STD FREE ENERGY OF ANION FORMATION: -278.8 KCAL FOR AQ SOLN @ 25 °C, Density: 1.542 g/cu cm /Citric acid monohydrate/, White, odorless crystals, granules or powder; cool, saline taste. Stable in air, becomes anhydrous at 150 °C. Density: 1.814. Soluble in 3 parts water, 0.6 parts boiling water. Insoluble in alcohol. The aqueous solution is slightly alkaline to litmus. pH about 8 /Sodium citrate dihydrate/ | |
| Record name | CITRIC ACID | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/10899 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | CITRIC ACID | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/911 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Mechanism of Action |
... The NK(2), and to a lesser extent the NK(1), receptors have been shown to be involved with citric acid-induced bronchoconstriction in the guinea pig, which is in part mediated by endogenously released bradykinin. Tachykinins and bradykinin could also modulate citric acid-induced bronchoconstriction. ... Bronchoconstriction induced by citric acid inhalation in the guinea pig, mainly caused by the tachykinin NK(2) receptor, is counteracted by bronchoprotective NO after activation of bradykinin B(2) and tachykinin NK(1) receptors in airway epithelium., ... A concentration of 47.6 mmol/L of citric acid (pH 2.3) in water led to total cell death within three minutes of incubation /with gingival fibroblasts (GF)/. Media containing 23.8 mmol/L and 47.6 mmol/L of citric acid exerted strong cytotoxicity (47 to 90 per cent of cell death) and inhibited protein synthesis (IC50 = 0.28 per cent) of GF within three hours of incubation. Incubation of cells in a medium containing 11.9 mmol/L of citric acid also suppressed the attachment and spreading of fibroblasts on culture plates and Type I collagen, with 58 per cent and 22 per cent of inhibition, respectively. Culture medium supplemented with 11.9, 23.8 and 47.6 mmol/L of citric acid also led to extracellular acidosis by decreasing the pH value from 7.5 to 6.3, 5.2 and 3.8, respectively., Inhalation of citric acid (CA) causes airway constriction and coughing. To investigate the role of mast cells in CA-induced airway constriction and cough, three experiments using guinea pigs were carried out. In the first experiment, /the authors/ used compound 48/80 to deplete mast cells, cromolyn sodium to stabilize mast cells, MK-886 to inhibit synthesis of leukotrienes, pyrilamine to antagonize histamine H1 receptor, methysergide to antagonize serotonin receptor, and indomethacin to inhibit cyclooxygenase. In the second experiment, compound 48/80-pretreated animals were divided into 2 parts; the first one was used to test the role of exogenous leukotriene (LT) C4, while the second one to test the role of exogenous histamine. Decreases in respiratory compliance (Crs) and forced expiratory volume in 0.1 sec (FEV0.1) were used as indicators for airway constriction in anesthetized guinea pigs. CA-induced cough was recorded for 12 min using a barometric body plethysmograph in conscious animals. In the third experiment, the activation of mast cells upon CA inhalation was investigated by determining lung tissue or arterial plasma histamine concentration in animals. Exposure to CA induced marked airway constriction and increase in cough number. Compound 48/80, cromolyn sodium, MK-886 and pyrilamine, but not indomethacin or methysergide, significantly attenuated CA-induced airway constriction and cough. Injection of LTC4 or histamine caused a significant increase in CA-induced airway constriction and cough in compound 48/80-pretreated animals. In addition, CA inhalation caused significant increase in lung tissue and plasma histamine concentrations, which were blocked by compound 48/80 pretreatment. These results suggest that mast cells play an important role in CA aerosol inhalation-induced airway constriction and cough via perhaps mediators including LTs and histamine. | |
| Record name | CITRIC ACID | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/911 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Crystals; monoclinic holohedra; crystallizes from hot concentrated aqueous solution, Colorless, translucent crystals or powder, Rhombic crystals from water with 1 mol of water of crystallization | |
CAS No. |
77-92-9 | |
| Record name | CITRIC ACID | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/10899 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Citric acid | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=77-92-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Citric acid, anhydrous [USP:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000077929 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Citric acid | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB04272 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | citric acid | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=759606 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Citric acid, anhydrous | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=626579 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | citric acid | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=30279 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | 1,2,3-Propanetricarboxylic acid, 2-hydroxy- | |
| Source | EPA Chemicals under the TSCA | |
| URL | https://www.epa.gov/chemicals-under-tsca | |
| Description | EPA Chemicals under the Toxic Substances Control Act (TSCA) collection contains information on chemicals and their regulations under TSCA, including non-confidential content from the TSCA Chemical Substance Inventory and Chemical Data Reporting. | |
| Record name | Citric acid | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3020332 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Citric acid | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.000.973 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | Citric acid | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | ANHYDROUS CITRIC ACID | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/XF417D3PSL | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | CITRIC ACID | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/911 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Citric acid | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0000094 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
| Record name | CITRIC ACID | |
| Source | ILO-WHO International Chemical Safety Cards (ICSCs) | |
| URL | https://www.ilo.org/dyn/icsc/showcard.display?p_version=2&p_card_id=0855 | |
| Description | The International Chemical Safety Cards (ICSCs) are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. The primary aim of the Cards is to promote the safe use of chemicals in the workplace. | |
| Explanation | Creative Commons CC BY 4.0 | |
Melting Point |
307 °F (anhydrous) (NTP, 1992), 153 °C | |
| Record name | CITRIC ACID | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/10899 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Citric acid | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB04272 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | CITRIC ACID | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/911 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Citric acid | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0000094 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
| Record name | CITRIC ACID | |
| Source | ILO-WHO International Chemical Safety Cards (ICSCs) | |
| URL | https://www.ilo.org/dyn/icsc/showcard.display?p_version=2&p_card_id=0855 | |
| Description | The International Chemical Safety Cards (ICSCs) are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. The primary aim of the Cards is to promote the safe use of chemicals in the workplace. | |
| Explanation | Creative Commons CC BY 4.0 | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.




![7-(Trifluoromethyl)imidazo[2,1-B]benzothiazole-2-carboxylic acid ethyl ester](/img/structure/B115153.png)
![4-[(4-Chlorophenyl)thio]thiophene-3-carboxylic acid](/img/structure/B115154.png)
![methyl 2-[(Z)-9-hydroxy-4,6-dimethyl-7-oxo-9-(3,4,5-trichloro-1H-pyrrol-2-yl)non-8-en-2-yl]-4,5-dihydro-1,3-thiazole-4-carboxylate](/img/structure/B115156.png)

![(Propane-1,3-diyl)bis[chloro(dimethyl)stannane]](/img/structure/B115173.png)