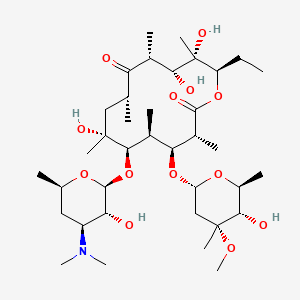
Erythromycin
Übersicht
Beschreibung
Erythromycin is a macrolide antibiotic that was first discovered in 1952This compound is widely used to treat a variety of bacterial infections, including respiratory tract infections, skin infections, chlamydia infections, pelvic inflammatory disease, and syphilis . It works by inhibiting bacterial protein synthesis, thereby preventing the growth of bacteria .
Wirkmechanismus
Target of Action
Erythromycin, a macrolide antibiotic, primarily targets bacterial ribosomal proteins . These proteins play a crucial role in protein synthesis, a process that is essential for bacterial growth and replication .
Mode of Action
This compound acts by inhibiting protein synthesis in bacteria . It binds to the 23S ribosomal RNA molecule in the 50S subunit of ribosomes in susceptible bacterial organisms . This binding blocks the process of transpeptidation, a critical step in protein synthesis . As a result, this compound prevents the growth of bacteria, making it a bacteriostatic antibiotic .
Biochemical Pathways
This compound affects the protein synthesis pathway in bacteria . By binding to the 23S ribosomal RNA molecule, it inhibits the transpeptidation process, thereby disrupting the elongation of the polypeptide chain . This disruption prevents the synthesis of essential proteins, affecting various biochemical pathways and functions within the bacterial cell .
Pharmacokinetics
This compound’s pharmacokinetic properties vary depending on the ester type, with bioavailability ranging between 30% and 65% . It is metabolized in the liver, with less than 5% excreted unchanged . The elimination half-life is approximately 1.5 hours under normal conditions, but can extend to 5 hours in anuria . This compound is distributed widely in the body, except to the brain and cerebrospinal fluid (CSF) . It is excreted in bile .
Result of Action
The primary result of this compound’s action is the inhibition of bacterial growth . By preventing protein synthesis, this compound disrupts various cellular functions, leading to the cessation of bacterial replication . This makes this compound effective in treating a variety of infections caused by susceptible strains of bacteria .
Action Environment
This compound’s action can be influenced by various environmental factors. For instance, its stability and efficacy can be affected by the pH of the environment . This compound is degraded in low pH environments, such as in the stomach . Therefore, it must be enteric coated for oral administration . Furthermore, this compound’s interaction with the cytochrome P450 system can affect its levels and the levels of other drugs metabolised by this system .
Wissenschaftliche Forschungsanwendungen
Erythromycin hat eine breite Palette von Anwendungen in der wissenschaftlichen Forschung:
Chemie: this compound wird als Ausgangsmaterial für die Synthese anderer Makrolid-Antibiotika verwendet.
Biologie: In der biologischen Forschung wird this compound verwendet, um die bakterielle Proteinbiosynthese und die Mechanismen der Antibiotikaresistenz zu untersuchen.
Medizin: this compound wird in klinischen Umgebungen häufig zur Behandlung bakterieller Infektionen eingesetzt.
5. Wirkmechanismus
This compound übt seine Wirkung aus, indem es an die 50S-ribosomale Untereinheit von Bakterien bindet und so die Proteinbiosynthese hemmt. Diese Wirkung verhindert die Verlängerung der Peptidkette und stoppt so das Wachstum von Bakterien. Das primäre molekulare Ziel von this compound ist das bakterielle Ribosom, und sein Wirkmechanismus beinhaltet das Blockieren des Austritstunnels, durch den neu synthetisierte Proteine hindurchgehen .
Biochemische Analyse
Biochemical Properties
Erythromycin plays a crucial role in biochemical reactions by interacting with various enzymes, proteins, and other biomolecules. It primarily targets the bacterial ribosome, specifically binding to the 23S ribosomal RNA within the 50S subunit. This binding inhibits the translocation of peptides, effectively halting protein synthesis. This compound also interacts with cytochrome P450 enzymes in the liver, which are involved in its metabolism .
Cellular Effects
This compound affects various types of cells and cellular processes. In bacterial cells, it inhibits protein synthesis by binding to the ribosome, leading to cell death. In eukaryotic cells, this compound can influence cell signaling pathways, gene expression, and cellular metabolism. For example, it has been shown to modulate the expression of genes involved in inflammatory responses and can affect mitochondrial function by inhibiting mitochondrial protein synthesis .
Molecular Mechanism
The molecular mechanism of this compound involves its binding to the 23S ribosomal RNA in the 50S subunit of the bacterial ribosome. This binding blocks the exit tunnel through which nascent peptides exit the ribosome, thereby inhibiting peptide chain elongation and protein synthesis. This compound’s interaction with the ribosome is highly specific, and its efficacy is influenced by the presence of resistance genes that can modify the ribosomal binding site .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of this compound can change over time. This compound is relatively stable under neutral pH but can degrade in acidic conditions. Long-term exposure to this compound in vitro can lead to the development of bacterial resistance, characterized by mutations in the ribosomal RNA or the acquisition of resistance genes. Additionally, this compound’s stability and efficacy can be influenced by storage conditions and the presence of other compounds .
Dosage Effects in Animal Models
The effects of this compound vary with different dosages in animal models. At therapeutic doses, this compound effectively treats bacterial infections without significant adverse effects. At higher doses, it can cause gastrointestinal disturbances, hepatotoxicity, and cardiotoxicity. In animal studies, this compound has been shown to have a dose-dependent effect on bacterial clearance and the development of resistance .
Metabolic Pathways
This compound is metabolized primarily in the liver by cytochrome P450 enzymes, particularly CYP3A4. The metabolic pathways involve demethylation and hydrolysis, resulting in various metabolites that are excreted in the bile. This compound can also affect the metabolism of other drugs by inhibiting CYP3A4, leading to potential drug-drug interactions .
Transport and Distribution
This compound is transported and distributed within cells and tissues through passive diffusion and active transport mechanisms. It is highly protein-bound in the plasma and can accumulate in tissues such as the liver, lungs, and spleen. This compound’s distribution is influenced by its lipophilicity, allowing it to penetrate cell membranes and reach intracellular targets .
Subcellular Localization
This compound’s subcellular localization is primarily within the cytoplasm, where it exerts its antibacterial effects by targeting the ribosome. It can also localize to the mitochondria in eukaryotic cells, affecting mitochondrial protein synthesis. The localization of this compound is influenced by its chemical structure and the presence of specific transporters and binding proteins .
Vorbereitungsmethoden
Synthetische Routen und Reaktionsbedingungen: Erythromycin wird typischerweise durch ein Fermentationsprozess unter Verwendung des Bakteriums Saccharopolyspora erythraea synthetisiert. Der Fermentationsprozess beinhaltet das Wachstum des Bakteriums in einem nährstoffreichen Medium, das zur Produktion von this compound führt. Die Verbindung wird dann durch verschiedene chemische Verfahren extrahiert und gereinigt .
Industrielle Produktionsmethoden: In industriellen Umgebungen wird this compound im großen Maßstab unter Verwendung von Fermentationsbehältern hergestellt. Der Fermentationsprozess wird sorgfältig gesteuert, um die Ausbeute an this compound zu optimieren. Nach der Fermentation wird die Verbindung mit Lösungsmitteln extrahiert und durch Kristallisations- und Filtrationstechniken gereinigt .
Analyse Chemischer Reaktionen
Arten von Reaktionen: Erythromycin unterliegt mehreren Arten von chemischen Reaktionen, darunter Oxidations-, Reduktions- und Substitutionsreaktionen. Diese Reaktionen sind wichtig, um die Struktur von this compound zu modifizieren, um Derivate mit verbesserten pharmakologischen Eigenschaften zu erhalten .
Häufige Reagenzien und Bedingungen:
Oxidation: this compound kann unter kontrollierten Bedingungen mit Reagenzien wie Wasserstoffperoxid oder Kaliumpermanganat oxidiert werden.
Reduktion: Reduktionsreaktionen können mit Reagenzien wie Natriumborhydrid oder Lithiumaluminiumhydrid durchgeführt werden.
Hauptprodukte, die gebildet werden: Die Hauptprodukte, die aus diesen Reaktionen gebildet werden, umfassen verschiedene this compound-Derivate, wie z. B. Azithromycin, Clarithromycin und Roxithromycin. Diese Derivate wurden entwickelt, um einige der Einschränkungen von this compound zu überwinden, wie z. B. die schlechte Stabilität in sauren Bedingungen .
Vergleich Mit ähnlichen Verbindungen
Erythromycin gehört zur Klasse der Makrolid-Antibiotika, zu der auch andere Verbindungen wie Azithromycin, Clarithromycin und Roxithromycin gehören. Diese Verbindungen haben einen ähnlichen Wirkmechanismus, unterscheiden sich jedoch in ihren pharmakokinetischen Eigenschaften und ihrem Wirkungsspektrum .
Ähnliche Verbindungen:
Azithromycin: Bekannt für seine verlängerte Halbwertszeit und verbesserte Gewebsdurchdringung.
Clarithromycin: Bemerkenswert für seine verbesserte Aktivität gegen bestimmte Bakterienstämme und verbesserte Säurestabilität.
Roxithromycin: Entwickelt, um eine bessere gastrointestinale Verträglichkeit und eine längere Halbwertszeit im Vergleich zu this compound zu bieten.
This compound ist ein wertvolles Antibiotikum aufgrund seines breiten Wirkungsspektrums und seiner Rolle als Vorläufer für die Entwicklung anderer Makrolid-Antibiotika.
Eigenschaften
IUPAC Name |
(3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-6-[(2S,3R,4S,6R)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-14-ethyl-7,12,13-trihydroxy-4-[(2R,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyloxan-2-yl]oxy-3,5,7,9,11,13-hexamethyl-oxacyclotetradecane-2,10-dione | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C37H67NO13/c1-14-25-37(10,45)30(41)20(4)27(39)18(2)16-35(8,44)32(51-34-28(40)24(38(11)12)15-19(3)47-34)21(5)29(22(6)33(43)49-25)50-26-17-36(9,46-13)31(42)23(7)48-26/h18-26,28-32,34,40-42,44-45H,14-17H2,1-13H3/t18-,19-,20+,21+,22-,23+,24+,25-,26+,28-,29+,30-,31+,32-,34+,35-,36-,37-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
ULGZDMOVFRHVEP-RWJQBGPGSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCC1C(C(C(C(=O)C(CC(C(C(C(C(C(=O)O1)C)OC2CC(C(C(O2)C)O)(C)OC)C)OC3C(C(CC(O3)C)N(C)C)O)(C)O)C)C)O)(C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC[C@@H]1[C@@]([C@@H]([C@H](C(=O)[C@@H](C[C@@]([C@@H]([C@H]([C@@H]([C@H](C(=O)O1)C)O[C@H]2C[C@@]([C@H]([C@@H](O2)C)O)(C)OC)C)O[C@H]3[C@@H]([C@H](C[C@H](O3)C)N(C)C)O)(C)O)C)C)O)(C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C37H67NO13 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID4022991 | |
| Record name | Erythromycin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID4022991 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
733.9 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Erythromycin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014344 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Soluble in water at 2mg/ml, WHITE OR SLIGHTLY YELLOW CRYSTALS OR POWDER, PRACTICALLY ODORLESS. SOLN IS ALKALINE TO LITMUS. SOL IN METHANOL, CHLOROFORM. /Erythromycin stearate/, WHITE OR SLIGHTLY YELLOW, CRYSTALLINE POWDER. ODORLESS OR PRACTICALLY SO. PRACTICALLY TASTELESS. PKA 7. FREELY SOL IN ACETONE & CHLOROFORM; SOL IN 95% ETHANOL & BENZENE; SPARINGLY SOL IN ETHER; VERY SLIGHTLY SOL IN WATER. /Erythromycin ethyl succinate/, FREELY SOLUBLE IN ALC, SOL IN POLYETHYLENE GLYCOL /Erythromycin ethyl succinate, Very soluble in acetone, ethyl ether, ethanol, chloroform, Freely soluble in alcohols, acetone, chloroform, acetonitrile, ethyl acetate; moderately soluble in ether, ethylene dichloride, amyl acetate, Solubility in water: approx 2 mg/ML, 4.59e-01 g/L | |
| Record name | Erythromycin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00199 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Erythromycin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3074 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Erythromycin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014344 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
In order to replicate, bacteria require a specific process of protein synthesis, enabled by ribosomal proteins. Erythromycin acts by inhibition of protein synthesis by binding to the 23S ribosomal RNA molecule in the 50S subunit of ribosomes in susceptible bacterial organisms. It stops bacterial protein synthesis by inhibiting the transpeptidation/translocation step of protein synthesis and by inhibiting the assembly of the 50S ribosomal subunit. This results in the control of various bacterial infections. The strong affinity of macrolides, including erythromycin, for bacterial ribosomes, supports their broad‐spectrum antibacterial activities., Macrolide antibiotics are bacteriostatic agents that inhibit protein synthesis by binding reversibly to 50S ribosomal subunits of sensitive microorganisms, at or very near the site that binds chloramphenicol. Erythromycin does not inhibit peptide bond formation per se, but rather inhibits the translocation step wherein a newly synthesized peptidyl tRNA molecule moves from the acceptor site on the ribosome to the peptidyl donor site. Gram-positive bacteria accumulate about 100 times more erythromycin than do gram-negative bacteria. Cells are considerably more permeable to the un-ionized form of the drug, which probably explains the increased antimicrobial activity at alkaline pH., ... /Erythromycin/ inhibits the growth of susceptible organisms (principally Propionibacterium acnes) on the surface of the skin and reduces the concn of free fatty acids in sebum ... The reduction in free fatty acids in sebum may be an indirect result of the inhibition of lipase-producing organisms which convert triglycerides into free fatty acids or may be a direct result of interference with lipase production in these organisms. /In acne treatment regimens/, Although stromal-derived factor-1 (SDF-1) via its cognate receptor CXCR4 is assumed to play a critical role in migration of endothelial cells during new vessel formation after tissue injury, CXCR4 expression on endothelial cells is strictly regulated. Erythromycin (EM), a 14-membered ring macrolide, has an anti-inflammatory effect that may account for its clinical benefit in the treatment of chronic inflammatory diseases. However, the effects of EM on endothelial cells and especially their expression of CXCR4 have not been fully evaluated. In this study, we demonstrated that EM markedly induced CXCR4 surface expression on microvascular endothelial cells in vitro and lung capillary endothelial cells in vivo. This ability to induce CXCR4 surface expression on endothelial cells was restricted to 14-membered ring macrolides and was not observed in other antibiotics including a 16-membered ring macrolide, josamycin. Furthermore, this EM-induced expression of CXCR4 on endothelial cells was functionally significant as demonstrated by chemotaxis assays in vitro. These findings suggest that EM-induced CXCR4 surface expression on endothelial cells may promote migration of CXCR4-expressing endothelial cells into sites of tissue injury, which may be associated with the known anti-inflammatory activity of this macrolide. | |
| Record name | Erythromycin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00199 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Erythromycin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3074 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Hydrated crystals from water, Crystals from water, White or slightly yellow crystals or powder | |
CAS No. |
114-07-8, 82343-12-2, 215031-94-0, 7540-22-9 | |
| Record name | Erythromycin | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=114-07-8 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Erythromycin [USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000114078 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | N-Methylerythromycin A | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0082343122 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Erythromycin C-13 | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0215031940 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Erythromycin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00199 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | erythromycin | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=756759 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Erythromycin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID4022991 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Erythromycin | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.003.673 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | Erythromycin | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | Erythromycin | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/63937KV33D | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Erythromycin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3074 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Erythromycin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014344 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
133-135, 191 °C, After melting /at 135-140 °C, it/ resolidifies with second melting point 190-193 °C. ... Readily forms salts with acids, MP: 92 °C. Slightly soluble in ethanol, ethyl ether, chloroform; insoluble in water. /Erythromycin stearate/, Crystals from acetone aqueous. MP: 222 °C. MW: 862.05. /Erythromycin ethyl succinate/ | |
| Record name | Erythromycin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00199 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Erythromycin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3074 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Erythromycin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014344 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.


![2-(3,4-dichlorophenyl)-N-[(1R,2R)-5-methoxy-2-pyrrolidin-1-yl-1,2,3,4-tetrahydronaphthalen-1-yl]-N-methylacetamide](/img/structure/B1670993.png)
![N-BENZYL-N'-[3-(TRIMETHOXYSILYL)PROPYL]ETHYLENEDIAMINE MONOHYDROCHLORIDE](/img/structure/B1671004.png)
