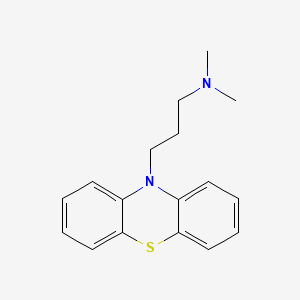
Promazin
Übersicht
Beschreibung
Promazin ist ein Phenothiazin-Derivat, das hauptsächlich als Beruhigungsmittel in der Veterinärmedizin und zur kurzfristigen Behandlung von psychomotorischer Unruhe beim Menschen eingesetzt wird . Es hat schwache antipsychotische Wirkungen und wird nicht häufig zur Behandlung von Psychosen eingesetzt . This compound wirkt ähnlich wie Chlorthis compound und führt zu Sedierung . Es gehört zur Klasse der typischen Antipsychotika und Phenothiazine .
Herstellungsmethoden
This compound kann durch verschiedene Verfahren synthetisiert werden. Eine gängige Methode beinhaltet die Reaktion von Diethylamin und Epoxypropan, um 1-Diethylamino-2-propanol zu erhalten, das dann mit Chlorsulfoxid und Toluol zu 1-Diethylamino-2-chlorpropan umgesetzt wird . Dieses Zwischenprodukt wird dann mit Phenothiazin umgesetzt, um rohes this compound zu erhalten, das gereinigt und mit Salzsäure zu Promazinhydrochlorid verestert wird . Industrielle Produktionsverfahren beinhalten oft die Kontrolle der Dosierung von Rohstoffen, der Reaktionstemperatur und des pH-Wertes, um eine hohe Ausbeute und Reinheit zu erzielen .
Wirkmechanismus
Target of Action
Promazine, a phenothiazine antipsychotic, primarily targets a variety of receptors in the brain . Its primary targets include dopamine receptors (types 1, 2, and 4) , serotonin (5-HT) receptor types 2A and 2C , muscarinic receptors 1 through 5 , alpha(1)-receptors , and histamine H1-receptors . These receptors play crucial roles in transmitting signals between brain cells .
Mode of Action
Promazine acts as an antagonist at its target receptors . This means it binds to these receptors and inhibits their activity. In particular, it blocks dopamine receptors, which are involved in transmitting signals between brain cells . When there is an excess amount of dopamine in the brain, it can cause over-stimulation of dopamine receptors . By blocking these receptors, Promazine helps to regulate this signal transmission .
Biochemical Pathways
It is known that the drug’s antagonistic action on dopamine and serotonin receptors can affect various neural pathways and alter neurotransmission
Pharmacokinetics
It is known that promazine is a small molecule , which suggests it may be well-absorbed and distributed throughout the body. The metabolism of Promazine is likely hepatic, as with other phenothiazines . More research is needed to fully understand the ADME properties of Promazine and their impact on its bioavailability.
Result of Action
The molecular and cellular effects of Promazine’s action primarily involve the regulation of neurotransmission in the brain. By blocking various receptors, Promazine can help to regulate the transmission of signals between brain cells . This can help to manage conditions such as schizophrenia and psychomotor agitation .
Action Environment
The action, efficacy, and stability of Promazine can be influenced by various environmental factors. For example, in veterinary medicine, Promazine is used as a tranquilizer and its efficacy can be influenced by the animal’s health status and other concurrent medications . .
Wissenschaftliche Forschungsanwendungen
Promazin hat mehrere Anwendungen in der wissenschaftlichen Forschung. Es wird zur Behandlung von psychomotorischer Unruhe und als Beruhigungsmittel in der Veterinärmedizin eingesetzt . Darüber hinaus wird es in elektrochemischen Sensoren zur Überwachung von pharmazeutischen Verbindungen in biologischen Proben eingesetzt . This compound wird auch hinsichtlich seines potenziellen Einsatzes bei der Entfernung von Phenothiazinen aus Umweltproben unter Verwendung von Biokohle untersucht .
Wirkmechanismus
This compound entfaltet seine Wirkung durch Blockierung verschiedener Rezeptoren im Gehirn, insbesondere Dopaminrezeptoren . Dopamin ist an der Übertragung von Signalen zwischen Gehirnzellen beteiligt, und ein Überschuss an Dopamin kann zu einer Überstimulation von Dopaminrezeptoren führen . This compound wirkt als Antagonist an den Dopaminrezeptortypen 1, 2 und 4, den 5-HT-Rezeptortypen 2A und 2C, den muskarinischen Rezeptoren 1 bis 5, den Alpha(1)-Rezeptoren und den Histamin-H1-Rezeptoren .
Biochemische Analyse
Biochemical Properties
Promazine acts by blocking a variety of receptors in the brain, particularly dopamine receptors . Dopamine is involved in transmitting signals between brain cells . When there is an excess amount of dopamine in the brain, it causes over-stimulation of dopamine receptors .
Cellular Effects
Promazine has weak extrapyramidal and autonomic side effects which lead to its use in the elderly, for restless or psychotic patients .
Molecular Mechanism
The mechanism of action of Promazine involves antagonism at types 1, 2, and 4 dopamine receptors, 5-HT receptor types 2A and 2C, muscarinic receptors 1 through 5, alpha(1)-receptors, and histamine H1-receptors .
Temporal Effects in Laboratory Settings
Promazine is subject to considerable first-pass metabolism, resulting in significant plasma concentration variations between patients . It is highly toxic in overdose and can result in grand mal seizures, QT prolongation, and comas .
Dosage Effects in Animal Models
In large male animals, protrusion of the penis may occur . Excitement, restlessness, sweating, trembling, and, rarely, seizures and recumbency may occur .
Metabolic Pathways
Three types of metabolic mechanisms were characterized, including S-oxidation, aromatic hydroxylation, and N-dealkylation . The calculated results demonstrate that N14-demethylation is the most thermodynamically and kinetically favorable metabolic pathway of Promazine, followed by S5-oxidation .
Vorbereitungsmethoden
Promazine can be synthesized through various methods. One common method involves reacting diethylamine and epoxypropane to obtain 1-diethylamino-2-propanol, which is then reacted with chloride sulfoxide and toluene to obtain 1-diethylamino-2-chloropropane . This intermediate is then reacted with phenothiazine to obtain crude promazine, which is purified and salified with hydrochloric acid to obtain promazine hydrochloride . Industrial production methods often involve controlling raw material dosage, reaction temperature, and pH value to achieve high yield and purity .
Analyse Chemischer Reaktionen
Promazin unterliegt verschiedenen chemischen Reaktionen, darunter Oxidation, Reduktion und Substitution. Zum Beispiel kann es mit Natrium-N-Brom-benzolsulfonamid in saurem Medium zu this compound-S-oxid oxidiert werden . Häufig verwendete Reagenzien in diesen Reaktionen sind Salzsäure, Methanol und Aktivkohle . Die Hauptprodukte, die aus diesen Reaktionen gebildet werden, sind this compound-S-oxid und andere oxidierte Derivate .
Vergleich Mit ähnlichen Verbindungen
Promazin ähnelt anderen Phenothiazin-Derivaten wie Chlorthis compound und Promethazin . Es hat schwächere antipsychotische Wirkungen im Vergleich zu Chlorthis compound . This compound und Promethazin binden beide an GDP-KRAS, aber this compound hat eine höhere Affinität . Andere ähnliche Verbindungen sind Cariprazin und Quetiapin, atypische Antipsychotika, die zur Behandlung von Schizophrenie und bipolarer Störung eingesetzt werden .
Eigenschaften
IUPAC Name |
N,N-dimethyl-3-phenothiazin-10-ylpropan-1-amine | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C17H20N2S/c1-18(2)12-7-13-19-14-8-3-5-10-16(14)20-17-11-6-4-9-15(17)19/h3-6,8-11H,7,12-13H2,1-2H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
ZGUGWUXLJSTTMA-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CN(C)CCCN1C2=CC=CC=C2SC3=CC=CC=C31 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C17H20N2S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID2023517 | |
| Record name | Promazine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2023517 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
284.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Promazine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014564 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Boiling Point |
203-210 °C at 3.00E-01 mm Hg, 203-210 °C @ 0.3 mm Hg | |
| Record name | Promazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00420 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | PROMAZINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3172 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Solubility |
Sol in methanol, ethanol, chloroform /Hydrochloride/, Practically insol in ether, benzene /Hydrochloride/, In water, 14.2 mg/l @ 24 °C, 2.07e-02 g/L | |
| Record name | Promazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00420 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | PROMAZINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3172 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Promazine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014564 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Promazine is an antagonist at types 1, 2, and 4 dopamine receptors, 5-HT receptor types 2A and 2C, muscarinic receptors 1 through 5, alpha(1)-receptors, and histamine H1-receptors. Promazine's antipsychotic effect is due to antagonism at dopamine and serotonin type 2 receptors, with greater activity at serotonin 5-HT2 receptors than at dopamine type-2 receptors. This may explain the lack of extrapyramidal effects. Promazine does not appear to block dopamine within the tubero-infundibular tract, explaining the lower incidence of hyperprolactinemia than with typical antipsychotic agents or risperidone. Antagonism at muscarinic receptors, H1-receptors, and alpha(1)-receptors also occurs with promazine., THERE IS AN ADENYLATE CYCLASE IN LIMBIC SYSTEM, AS WELL AS IN CAUDATE NUCLEUS, THAT IS SPECIFICALLY ACTIVATED BY DOPAMINE. ...ACTIVATION OF...ENZYME IS... BLOCKED BY...PHENOTHIAZINES. ...THERAPEUTIC EFFICACY & SIDE EFFECTS MAY RELATE TO INHIBITION OF DOPAMINE ACTIVATION OF ADENYLATE CYCLASE. /PHENOTHIAZINES/, ...PHENOTHIAZINES, BLOCK DOPAMINE RECEPTORS & INCR TURNOVER RATE OF DOPAMINE IN CORPUS STRIATUM. INCR TURNOVER RATE IS BELIEVED TO BE RESULT OF NEURONAL FEEDBACK MECHANISM. ...FIRING OF.../IDENTIFIED DOPAMINERGIC NEURONS IN SUBSTANTIA NIGRA & VENTRAL TEGMENTAL AREAS/ IS INCR BY ANTIPSYCHOTIC PHENOTHIAZINES. /PHENOTHIAZINES/ | |
| Record name | Promazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00420 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | PROMAZINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3172 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Oily liq | |
CAS No. |
58-40-2 | |
| Record name | Promazine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=58-40-2 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Promazine [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000058402 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Promazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00420 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | promazine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=31447 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Promazine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2023517 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Promazine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.000.347 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | PROMAZINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/O9M39HTM5W | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | PROMAZINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3172 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Promazine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014564 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
< 25 °C | |
| Record name | Promazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00420 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Promazine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014564 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary mechanism of action of Promazine?
A1: While the precise mechanism of action remains an area of ongoing research, Promazine is understood to primarily act as a dopamine antagonist. It binds to dopamine receptors, particularly D2 receptors, in the brain, thereby blocking the action of dopamine. [] This dopamine antagonism is thought to contribute to its antipsychotic effects. []
Q2: Does Promazine affect other neurotransmitter systems?
A2: Yes, in addition to its effects on dopamine receptors, Promazine also interacts with adrenergic receptors, demonstrating a strong antiadrenergic action. [] This interaction contributes to some of its side effects, including potential for faintness and palpitation. []
Q3: What is the molecular formula and weight of Promazine?
A3: Promazine's molecular formula is C17H20N2S, and its molecular weight is 284.42 g/mol.
Q4: What are the key structural differences between Promazine and Chlorpromazine?
A4: Promazine and Chlorpromazine share the same basic phenothiazine structure. The key difference lies in the absence of a chlorine atom at the 2-position of the phenothiazine ring in Promazine. This subtle structural variation contributes to the differences observed in their pharmacological profiles. [, , ]
Q5: Is there spectroscopic data available for Promazine and its metabolites?
A5: Yes, various spectroscopic techniques, including mass spectrometry and UV spectroscopy, have been employed to characterize Promazine and its metabolites. For example, mass spectral analysis has been used to identify the dimeric product and 2-chlorophenothiazine sulfoxide formed during the photodecomposition of Chlorpromazine. [] Additionally, UV spectroscopy has been utilized to differentiate between Promazine, Chlorpromazine, and their respective sulfoxide metabolites based on their unique absorption spectra. []
Q6: How is Promazine metabolized in the body?
A6: Promazine undergoes extensive metabolism in the liver, primarily via hydroxylation followed by conjugation with glucuronic acid and, to a lesser extent, with sulfuric acid. [] These metabolic processes lead to the formation of numerous metabolites, with over 30 different metabolites identified in horse urine. []
Q7: Does the presence of liver disease affect Promazine pharmacokinetics?
A7: Yes, studies in patients with hepatic cirrhosis have shown significant alterations in Promazine pharmacokinetics. [] These alterations include reduced total plasma clearance, reduced free drug clearance, reduced metabolic clearance, and increased elimination half-life. [] These findings highlight the importance of careful dosage adjustments in patients with liver cirrhosis.
Q8: How does the route of administration affect Promazine's pharmacokinetic profile?
A8: The route of administration significantly influences the pharmacokinetics of Promazine. While intravenous administration results in rapid absorption and distribution, oral and sublingual administration lead to slower absorption and prolonged elimination half-lives. [] Furthermore, the proportion of different metabolites excreted in the urine may vary depending on the route of administration. []
Q9: What in vitro models have been used to study Promazine's effects?
A9: Promazine's effects have been investigated using various in vitro models, including isolated rat atria. [] In these models, Promazine has been shown to influence both contractile force and rate, as well as modulate the response to noradrenaline. [] Such studies provide insights into the direct effects of Promazine on cardiac tissue.
Q10: What animal models have been used to study Promazine?
A10: Promazine's effects have been extensively studied in various animal models, including rats, mice, horses, and pigeons. [, , , ] These models have been instrumental in understanding the drug's behavioral effects, metabolic pathways, and potential toxicity. For example, studies in horses have revealed the extensive metabolism of Promazine, while studies in pigeons have provided insights into its effects on operant conditioning paradigms. [, ]
Q11: What are some of the known side effects of Promazine?
A11: Promazine, like other phenothiazine derivatives, can induce a range of side effects. Some of the commonly reported side effects include drowsiness, dizziness, postural hypotension, and Parkinsonian symptoms. [] In rare instances, more serious adverse effects such as agranulocytosis and seizures have been reported. [, ]
Q12: What analytical techniques are commonly employed to measure Promazine levels?
A12: Various analytical methods have been developed for the detection and quantification of Promazine in biological samples. Gas chromatography (GC) coupled with nitrogen-phosphorus detection (NPD) is a highly sensitive and selective method that allows for the simultaneous determination of Promazine and its metabolites in human blood and plasma. [] High-performance liquid chromatography (HPLC) coupled with tandem mass spectrometry (MS/MS) offers another powerful approach for analyzing Promazine and its metabolites in various matrices. []
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.


![(3R)-3-[[(2S)-2-(2-adamantyloxycarbonylamino)-3-(1H-indol-3-yl)-2-methylpropanoyl]amino]-4-phenylbutanoic acid](/img/structure/B1679105.png)
![tert-butyl N-[(2S)-1-[[(2R)-2-(8-hydroxyoctylamino)-2-methyl-3-phenylpropanoyl]amino]-1-oxo-3-phenylpropan-2-yl]carbamate](/img/structure/B1679114.png)
![[(1S)-2-methyl-1-phenylpropyl] N-[(2S)-1-[7-(carbamoylamino)heptylamino]-3-(2,3-difluorophenyl)-2-methyl-1-oxopropan-2-yl]carbamate](/img/structure/B1679119.png)
![(2S)-2-[[2,6-di(propan-2-yl)phenyl]carbamoylamino]-3-(1H-indol-3-yl)-2-methyl-N-[(1-pyridin-2-ylcyclohexyl)methyl]propanamide](/img/structure/B1679121.png)
![6-(2,6-dichlorophenyl)-2-[4-[2-(diethylamino)ethoxy]anilino]-8-methylpyrido[2,3-d]pyrimidin-7-one;dihydrochloride](/img/structure/B1679122.png)
