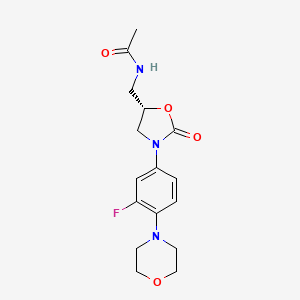
Linezolid
Katalognummer B1675486
Molekulargewicht: 337.35 g/mol
InChI-Schlüssel: TYZROVQLWOKYKF-ZDUSSCGKSA-N
Achtung: Nur für Forschungszwecke. Nicht für den menschlichen oder tierärztlichen Gebrauch.
Patent
US06362334B1
Procedure details


To a solution of (±)-glycidylacetamide (VIIIB, EXAMPLE 11, 0.1571 g, 1.365 mmol) in THF (1.63 ml) at −78° is added N-carbomethoxy-3-fluoro-4-morpholinylaniline (IX, PREPARATION 2, 0.4358 g, 1.71 mmol, 1.26 eq) and lithium t-butoxide (0.1267 g, 1.583 mmol, 1.16 eq). The reaction mixture is then stirred at 0 to 11° for 17.5 hrs at which point HPLC showes an 80% yield of (±)-N-[[3-(3-fluoro-4-morpholinylphenyl)-2-oxo-5-oxazolidinyl]methyl]acetamide (retention time=0.97 min; method B; Stationary phase: 4.6×250 mm Zorbax RX C-8 column; mobile phase: 650 ml acetonitrile, 1.85 ml triethylamine, 1.30 ml acetic acid, water sufficient to make 1000 ml; flow rate: 3.0 ml/min; UV detection at 254 nm). The title compound is isolated by means known to those skilled in the art.
Name
(±)-glycidylacetamide
Quantity
0.1571 g
Type
reactant
Reaction Step One


Name
lithium t-butoxide
Quantity
0.1267 g
Type
reactant
Reaction Step One


Identifiers


|
REACTION_CXSMILES
|
C([CH2:5][C:6]([NH2:8])=[O:7])C1OC1.[C:9]([NH:13][C:14]1[CH:19]=[CH:18][C:17]([N:20]2[CH2:25][CH2:24][O:23][CH2:22][CH2:21]2)=[C:16]([F:26])[CH:15]=1)([O:11]C)=O.[CH3:27][C:28](C)([O-:30])[CH3:29].[Li+]>C1COCC1>[F:26][C:16]1[CH:15]=[C:14]([N:13]2[CH2:27][CH:28]([CH2:29][NH:8][C:6](=[O:7])[CH3:5])[O:30][C:9]2=[O:11])[CH:19]=[CH:18][C:17]=1[N:20]1[CH2:25][CH2:24][O:23][CH2:22][CH2:21]1 |f:2.3|
|
Inputs


Step One
|
Name
|
(±)-glycidylacetamide
|
|
Quantity
|
0.1571 g
|
|
Type
|
reactant
|
|
Smiles
|
C(C1CO1)CC(=O)N
|
|
Name
|
|
|
Quantity
|
0.4358 g
|
|
Type
|
reactant
|
|
Smiles
|
C(=O)(OC)NC1=CC(=C(C=C1)N1CCOCC1)F
|
|
Name
|
lithium t-butoxide
|
|
Quantity
|
0.1267 g
|
|
Type
|
reactant
|
|
Smiles
|
CC(C)([O-])C.[Li+]
|
|
Name
|
|
|
Quantity
|
1.63 mL
|
|
Type
|
solvent
|
|
Smiles
|
C1CCOC1
|
Conditions


Stirring
|
Type
|
CUSTOM
|
|
Details
|
The reaction mixture is then stirred at 0 to 11° for 17.5 hrs at which point HPLC
|
|
Rate
|
UNSPECIFIED
|
|
RPM
|
0
|
Other
|
Conditions are dynamic
|
1
|
|
Details
|
See reaction.notes.procedure_details.
|
Outcomes


Product
Details
Reaction Time |
17.5 h |
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
FC=1C=C(C=CC1N1CCOCC1)N1C(OC(C1)CNC(C)=O)=O
|
Measurements
| Type | Value | Analysis |
|---|---|---|
| AMOUNT: VOLUME | 650 mL | |
| YIELD: PERCENTYIELD | 80% |
Source


|
Source
|
Open Reaction Database (ORD) |
|
Description
|
The Open Reaction Database (ORD) is an open-access schema and infrastructure for structuring and sharing organic reaction data, including a centralized data repository. The ORD schema supports conventional and emerging technologies, from benchtop reactions to automated high-throughput experiments and flow chemistry. Our vision is that a consistent data representation and infrastructure to support data sharing will enable downstream applications that will greatly improve the state of the art with respect to computer-aided synthesis planning, reaction prediction, and other predictive chemistry tasks. |
Patent
US06362334B1
Procedure details


To a solution of (±)-glycidylacetamide (VIIIB, EXAMPLE 11, 0.1571 g, 1.365 mmol) in THF (1.63 ml) at −78° is added N-carbomethoxy-3-fluoro-4-morpholinylaniline (IX, PREPARATION 2, 0.4358 g, 1.71 mmol, 1.26 eq) and lithium t-butoxide (0.1267 g, 1.583 mmol, 1.16 eq). The reaction mixture is then stirred at 0 to 11° for 17.5 hrs at which point HPLC showes an 80% yield of (±)-N-[[3-(3-fluoro-4-morpholinylphenyl)-2-oxo-5-oxazolidinyl]methyl]acetamide (retention time=0.97 min; method B; Stationary phase: 4.6×250 mm Zorbax RX C-8 column; mobile phase: 650 ml acetonitrile, 1.85 ml triethylamine, 1.30 ml acetic acid, water sufficient to make 1000 ml; flow rate: 3.0 ml/min; UV detection at 254 nm). The title compound is isolated by means known to those skilled in the art.
Name
(±)-glycidylacetamide
Quantity
0.1571 g
Type
reactant
Reaction Step One


Name
lithium t-butoxide
Quantity
0.1267 g
Type
reactant
Reaction Step One


Identifiers


|
REACTION_CXSMILES
|
C([CH2:5][C:6]([NH2:8])=[O:7])C1OC1.[C:9]([NH:13][C:14]1[CH:19]=[CH:18][C:17]([N:20]2[CH2:25][CH2:24][O:23][CH2:22][CH2:21]2)=[C:16]([F:26])[CH:15]=1)([O:11]C)=O.[CH3:27][C:28](C)([O-:30])[CH3:29].[Li+]>C1COCC1>[F:26][C:16]1[CH:15]=[C:14]([N:13]2[CH2:27][CH:28]([CH2:29][NH:8][C:6](=[O:7])[CH3:5])[O:30][C:9]2=[O:11])[CH:19]=[CH:18][C:17]=1[N:20]1[CH2:25][CH2:24][O:23][CH2:22][CH2:21]1 |f:2.3|
|
Inputs


Step One
|
Name
|
(±)-glycidylacetamide
|
|
Quantity
|
0.1571 g
|
|
Type
|
reactant
|
|
Smiles
|
C(C1CO1)CC(=O)N
|
|
Name
|
|
|
Quantity
|
0.4358 g
|
|
Type
|
reactant
|
|
Smiles
|
C(=O)(OC)NC1=CC(=C(C=C1)N1CCOCC1)F
|
|
Name
|
lithium t-butoxide
|
|
Quantity
|
0.1267 g
|
|
Type
|
reactant
|
|
Smiles
|
CC(C)([O-])C.[Li+]
|
|
Name
|
|
|
Quantity
|
1.63 mL
|
|
Type
|
solvent
|
|
Smiles
|
C1CCOC1
|
Conditions


Stirring
|
Type
|
CUSTOM
|
|
Details
|
The reaction mixture is then stirred at 0 to 11° for 17.5 hrs at which point HPLC
|
|
Rate
|
UNSPECIFIED
|
|
RPM
|
0
|
Other
|
Conditions are dynamic
|
1
|
|
Details
|
See reaction.notes.procedure_details.
|
Outcomes


Product
Details
Reaction Time |
17.5 h |
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
FC=1C=C(C=CC1N1CCOCC1)N1C(OC(C1)CNC(C)=O)=O
|
Measurements
| Type | Value | Analysis |
|---|---|---|
| AMOUNT: VOLUME | 650 mL | |
| YIELD: PERCENTYIELD | 80% |
Source


|
Source
|
Open Reaction Database (ORD) |
|
Description
|
The Open Reaction Database (ORD) is an open-access schema and infrastructure for structuring and sharing organic reaction data, including a centralized data repository. The ORD schema supports conventional and emerging technologies, from benchtop reactions to automated high-throughput experiments and flow chemistry. Our vision is that a consistent data representation and infrastructure to support data sharing will enable downstream applications that will greatly improve the state of the art with respect to computer-aided synthesis planning, reaction prediction, and other predictive chemistry tasks. |
Patent
US06362334B1
Procedure details


To a solution of (±)-glycidylacetamide (VIIIB, EXAMPLE 11, 0.1571 g, 1.365 mmol) in THF (1.63 ml) at −78° is added N-carbomethoxy-3-fluoro-4-morpholinylaniline (IX, PREPARATION 2, 0.4358 g, 1.71 mmol, 1.26 eq) and lithium t-butoxide (0.1267 g, 1.583 mmol, 1.16 eq). The reaction mixture is then stirred at 0 to 11° for 17.5 hrs at which point HPLC showes an 80% yield of (±)-N-[[3-(3-fluoro-4-morpholinylphenyl)-2-oxo-5-oxazolidinyl]methyl]acetamide (retention time=0.97 min; method B; Stationary phase: 4.6×250 mm Zorbax RX C-8 column; mobile phase: 650 ml acetonitrile, 1.85 ml triethylamine, 1.30 ml acetic acid, water sufficient to make 1000 ml; flow rate: 3.0 ml/min; UV detection at 254 nm). The title compound is isolated by means known to those skilled in the art.
Name
(±)-glycidylacetamide
Quantity
0.1571 g
Type
reactant
Reaction Step One


Name
lithium t-butoxide
Quantity
0.1267 g
Type
reactant
Reaction Step One


Identifiers


|
REACTION_CXSMILES
|
C([CH2:5][C:6]([NH2:8])=[O:7])C1OC1.[C:9]([NH:13][C:14]1[CH:19]=[CH:18][C:17]([N:20]2[CH2:25][CH2:24][O:23][CH2:22][CH2:21]2)=[C:16]([F:26])[CH:15]=1)([O:11]C)=O.[CH3:27][C:28](C)([O-:30])[CH3:29].[Li+]>C1COCC1>[F:26][C:16]1[CH:15]=[C:14]([N:13]2[CH2:27][CH:28]([CH2:29][NH:8][C:6](=[O:7])[CH3:5])[O:30][C:9]2=[O:11])[CH:19]=[CH:18][C:17]=1[N:20]1[CH2:25][CH2:24][O:23][CH2:22][CH2:21]1 |f:2.3|
|
Inputs


Step One
|
Name
|
(±)-glycidylacetamide
|
|
Quantity
|
0.1571 g
|
|
Type
|
reactant
|
|
Smiles
|
C(C1CO1)CC(=O)N
|
|
Name
|
|
|
Quantity
|
0.4358 g
|
|
Type
|
reactant
|
|
Smiles
|
C(=O)(OC)NC1=CC(=C(C=C1)N1CCOCC1)F
|
|
Name
|
lithium t-butoxide
|
|
Quantity
|
0.1267 g
|
|
Type
|
reactant
|
|
Smiles
|
CC(C)([O-])C.[Li+]
|
|
Name
|
|
|
Quantity
|
1.63 mL
|
|
Type
|
solvent
|
|
Smiles
|
C1CCOC1
|
Conditions


Stirring
|
Type
|
CUSTOM
|
|
Details
|
The reaction mixture is then stirred at 0 to 11° for 17.5 hrs at which point HPLC
|
|
Rate
|
UNSPECIFIED
|
|
RPM
|
0
|
Other
|
Conditions are dynamic
|
1
|
|
Details
|
See reaction.notes.procedure_details.
|
Outcomes


Product
Details
Reaction Time |
17.5 h |
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
FC=1C=C(C=CC1N1CCOCC1)N1C(OC(C1)CNC(C)=O)=O
|
Measurements
| Type | Value | Analysis |
|---|---|---|
| AMOUNT: VOLUME | 650 mL | |
| YIELD: PERCENTYIELD | 80% |
Source


|
Source
|
Open Reaction Database (ORD) |
|
Description
|
The Open Reaction Database (ORD) is an open-access schema and infrastructure for structuring and sharing organic reaction data, including a centralized data repository. The ORD schema supports conventional and emerging technologies, from benchtop reactions to automated high-throughput experiments and flow chemistry. Our vision is that a consistent data representation and infrastructure to support data sharing will enable downstream applications that will greatly improve the state of the art with respect to computer-aided synthesis planning, reaction prediction, and other predictive chemistry tasks. |
Patent
US06362334B1
Procedure details


To a solution of (±)-glycidylacetamide (VIIIB, EXAMPLE 11, 0.1571 g, 1.365 mmol) in THF (1.63 ml) at −78° is added N-carbomethoxy-3-fluoro-4-morpholinylaniline (IX, PREPARATION 2, 0.4358 g, 1.71 mmol, 1.26 eq) and lithium t-butoxide (0.1267 g, 1.583 mmol, 1.16 eq). The reaction mixture is then stirred at 0 to 11° for 17.5 hrs at which point HPLC showes an 80% yield of (±)-N-[[3-(3-fluoro-4-morpholinylphenyl)-2-oxo-5-oxazolidinyl]methyl]acetamide (retention time=0.97 min; method B; Stationary phase: 4.6×250 mm Zorbax RX C-8 column; mobile phase: 650 ml acetonitrile, 1.85 ml triethylamine, 1.30 ml acetic acid, water sufficient to make 1000 ml; flow rate: 3.0 ml/min; UV detection at 254 nm). The title compound is isolated by means known to those skilled in the art.
Name
(±)-glycidylacetamide
Quantity
0.1571 g
Type
reactant
Reaction Step One


Name
lithium t-butoxide
Quantity
0.1267 g
Type
reactant
Reaction Step One


Identifiers


|
REACTION_CXSMILES
|
C([CH2:5][C:6]([NH2:8])=[O:7])C1OC1.[C:9]([NH:13][C:14]1[CH:19]=[CH:18][C:17]([N:20]2[CH2:25][CH2:24][O:23][CH2:22][CH2:21]2)=[C:16]([F:26])[CH:15]=1)([O:11]C)=O.[CH3:27][C:28](C)([O-:30])[CH3:29].[Li+]>C1COCC1>[F:26][C:16]1[CH:15]=[C:14]([N:13]2[CH2:27][CH:28]([CH2:29][NH:8][C:6](=[O:7])[CH3:5])[O:30][C:9]2=[O:11])[CH:19]=[CH:18][C:17]=1[N:20]1[CH2:25][CH2:24][O:23][CH2:22][CH2:21]1 |f:2.3|
|
Inputs


Step One
|
Name
|
(±)-glycidylacetamide
|
|
Quantity
|
0.1571 g
|
|
Type
|
reactant
|
|
Smiles
|
C(C1CO1)CC(=O)N
|
|
Name
|
|
|
Quantity
|
0.4358 g
|
|
Type
|
reactant
|
|
Smiles
|
C(=O)(OC)NC1=CC(=C(C=C1)N1CCOCC1)F
|
|
Name
|
lithium t-butoxide
|
|
Quantity
|
0.1267 g
|
|
Type
|
reactant
|
|
Smiles
|
CC(C)([O-])C.[Li+]
|
|
Name
|
|
|
Quantity
|
1.63 mL
|
|
Type
|
solvent
|
|
Smiles
|
C1CCOC1
|
Conditions


Stirring
|
Type
|
CUSTOM
|
|
Details
|
The reaction mixture is then stirred at 0 to 11° for 17.5 hrs at which point HPLC
|
|
Rate
|
UNSPECIFIED
|
|
RPM
|
0
|
Other
|
Conditions are dynamic
|
1
|
|
Details
|
See reaction.notes.procedure_details.
|
Outcomes


Product
Details
Reaction Time |
17.5 h |
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
FC=1C=C(C=CC1N1CCOCC1)N1C(OC(C1)CNC(C)=O)=O
|
Measurements
| Type | Value | Analysis |
|---|---|---|
| AMOUNT: VOLUME | 650 mL | |
| YIELD: PERCENTYIELD | 80% |
Source


|
Source
|
Open Reaction Database (ORD) |
|
Description
|
The Open Reaction Database (ORD) is an open-access schema and infrastructure for structuring and sharing organic reaction data, including a centralized data repository. The ORD schema supports conventional and emerging technologies, from benchtop reactions to automated high-throughput experiments and flow chemistry. Our vision is that a consistent data representation and infrastructure to support data sharing will enable downstream applications that will greatly improve the state of the art with respect to computer-aided synthesis planning, reaction prediction, and other predictive chemistry tasks. |
