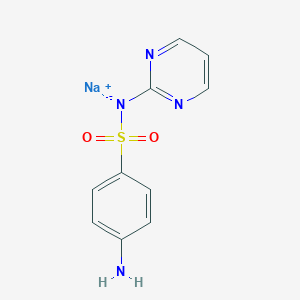
Sulfadiazine sodium
Katalognummer B000164
:
547-32-0
Molekulargewicht: 273.27 g/mol
InChI-Schlüssel: RKISJHYCSQKDTK-UHFFFAOYSA-N
Achtung: Nur für Forschungszwecke. Nicht für den menschlichen oder tierärztlichen Gebrauch.
Patent
US04608278
Procedure details


An aqueous solution consisting of 0.5 g sodium sulfadiazine, 0.5 ml ethyl alcohol, 13 ml of 20% sodium sulfate and 20 ml of 5% gelatin (type B: acid processed) was titrated, while under constant agitation with a magnetic stirrer, with 18.4 ml of 0.1N hydrochloric acid solution. This procedure resulted in a white suspension of microencapsulated sulfadiazine particles. The suspension was then stirred for an additional 15 minutes, following which it was poured into 200 ml of cold (5° C.) 7% sodium sulfate solution, and stirred for 30 minutes at ice-bath temperature. This procedure caused gelling of the liquid gelatin shell of the microcapsules. The entire process was monitored by observation of samples in the optical microscope. The microcapsules were of assymetric appearance and of a size less than 10 μm.
Name
sodium sulfadiazine
Quantity
0.5 g
Type
reactant
Reaction Step One



Identifiers


|
REACTION_CXSMILES
|
[CH:1]1[CH:6]=[N:5][C:4]([N-:7][S:8]([C:11]2[CH:16]=[CH:15][C:14]([NH2:17])=[CH:13][CH:12]=2)(=[O:10])=[O:9])=[N:3][CH:2]=1.[Na+].S([O-])([O-])(=O)=O.[Na+].[Na+].Cl>C(O)C>[CH:1]1[CH:2]=[N:3][C:4]([NH:7][S:8]([C:11]2[CH:12]=[CH:13][C:14]([NH2:17])=[CH:15][CH:16]=2)(=[O:10])=[O:9])=[N:5][CH:6]=1 |f:0.1,2.3.4|
|
Inputs


Step One
|
Name
|
sodium sulfadiazine
|
|
Quantity
|
0.5 g
|
|
Type
|
reactant
|
|
Smiles
|
C1=CN=C(N=C1)[N-]S(=O)(=O)C2=CC=C(C=C2)N.[Na+]
|
Step Two
|
Name
|
|
|
Quantity
|
13 mL
|
|
Type
|
reactant
|
|
Smiles
|
S(=O)(=O)([O-])[O-].[Na+].[Na+]
|
Step Three
|
Name
|
|
|
Quantity
|
18.4 mL
|
|
Type
|
reactant
|
|
Smiles
|
Cl
|
Step Four
|
Name
|
|
|
Quantity
|
0.5 mL
|
|
Type
|
solvent
|
|
Smiles
|
C(C)O
|
Conditions


Other
|
Conditions are dynamic
|
1
|
|
Details
|
See reaction.notes.procedure_details.
|
Outcomes


Product
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
C=1C=NC(=NC1)NS(=O)(=O)C=2C=CC(=CC2)N
|
Source


|
Source
|
Open Reaction Database (ORD) |
|
Description
|
The Open Reaction Database (ORD) is an open-access schema and infrastructure for structuring and sharing organic reaction data, including a centralized data repository. The ORD schema supports conventional and emerging technologies, from benchtop reactions to automated high-throughput experiments and flow chemistry. Our vision is that a consistent data representation and infrastructure to support data sharing will enable downstream applications that will greatly improve the state of the art with respect to computer-aided synthesis planning, reaction prediction, and other predictive chemistry tasks. |

