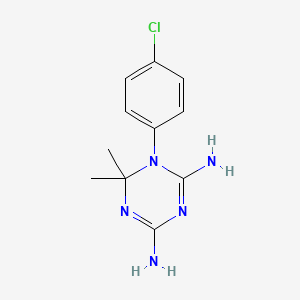
Cycloguanil
Overview
Description
Cycloguanil is a dihydrofolate reductase inhibitor and a metabolite of the antimalarial drug proguanil. It is primarily responsible for the antimalarial activity of proguanil.
Mechanism of Action
Target of Action
Cycloguanil primarily targets the dihydrofolate reductase (DHFR) enzyme . DHFR plays a crucial role in the synthesis of nucleic acids and amino acids, which are essential for cell growth and multiplication . By inhibiting DHFR, this compound disrupts these critical biological processes.
Mode of Action
This compound, as a DHFR inhibitor, binds to the active site of the DHFR enzyme, thereby preventing the enzyme from catalyzing the conversion of dihydrofolate to tetrahydrofolate . This inhibition disrupts the synthesis of nucleic acids and certain amino acids, leading to the cessation of cell growth and multiplication .
Biochemical Pathways
The primary biochemical pathway affected by this compound is the folate metabolism pathway . By inhibiting DHFR, this compound disrupts the conversion of dihydrofolate to tetrahydrofolate, a critical step in the folate metabolism pathway. Tetrahydrofolate is a vital cofactor in the synthesis of nucleic acids and certain amino acids. Therefore, the inhibition of DHFR leads to a decrease in the availability of tetrahydrofolate, disrupting these synthesis processes and ultimately leading to the cessation of cell growth and multiplication .
Pharmacokinetics
Proguanil, the prodrug of this compound, is rapidly and extensively absorbed, with its peak plasma concentration reached within 3 hours . It is then metabolized in the liver by the cytochrome P450 3A and 2C subfamilies to form this compound . The elimination half-lives of Proguanil and this compound are 12 to 15 hours in adults and children . This compound is predominantly eliminated unchanged in feces, with negligible excretion in urine .
Result of Action
The molecular effect of this compound’s action is the disruption of nucleic acid and amino acid synthesis due to the inhibition of DHFR . This leads to the cessation of cell growth and multiplication, which is the cellular effect of this compound’s action . In the context of malaria, this results in the death of the Plasmodium parasite, thereby treating the infection .
Action Environment
The action, efficacy, and stability of this compound can be influenced by various environmental factors. For instance, the presence of other drugs can lead to drug-drug interactions. As this compound is a substrate of organic cation transporters 1 and 2 (OCT1 and OCT2), and multidrug and toxin extrusion 1 and 2-K (MATE1 and MATE2-K), it may interact with other drugs that are substrates of these transporters . Such interactions could potentially affect the absorption, distribution, metabolism, and excretion of this compound, thereby influencing its action and efficacy .
Biochemical Analysis
Biochemical Properties
Cycloguanil plays a significant role in biochemical reactions, particularly in the inhibition of dihydrofolate reductase . This enzyme is crucial for the synthesis of nucleic acids, and its inhibition disrupts the reproduction of the malaria parasite . This compound interacts with this enzyme, leading to the disruption of deoxythymidylate synthesis .
Cellular Effects
This compound exerts its effects on various types of cells, particularly those infected by the malaria parasite. By inhibiting dihydrofolate reductase, it disrupts the parasite’s ability to reproduce within the host cell . This impacts cell function and can influence cell signaling pathways, gene expression, and cellular metabolism .
Molecular Mechanism
The molecular mechanism of this compound involves its binding to dihydrofolate reductase, inhibiting the enzyme and disrupting the synthesis of nucleic acids . This prevents the malaria parasite from reproducing within the host cell .
Metabolic Pathways
This compound is involved in the metabolic pathway related to the inhibition of dihydrofolate reductase . It interacts with this enzyme, disrupting the synthesis of nucleic acids and affecting metabolic flux and metabolite levels .
Transport and Distribution
This compound is transported and distributed within cells and tissues. It is known to be a substrate of organic cation transporters and multidrug and toxin extrusion proteins , which play a role in its distribution within cells.
Preparation Methods
Cycloguanil can be synthesized through a multi-step processThis intermediate is then condensed with acetone to produce this compound . Industrial production methods may involve optimizing reaction conditions to maximize yield and purity.
Chemical Reactions Analysis
Cycloguanil undergoes various chemical reactions, including:
Oxidation: this compound can be oxidized under specific conditions, leading to the formation of different oxidation products.
Reduction: Reduction reactions can convert this compound into its reduced forms.
Substitution: this compound can undergo substitution reactions, where specific functional groups are replaced by others.
Common reagents and conditions used in these reactions include oxidizing agents, reducing agents, and catalysts. The major products formed from these reactions depend on the specific reagents and conditions employed .
Scientific Research Applications
Cycloguanil has several scientific research applications, including:
Chemistry: this compound is used as a model compound in studying dihydrofolate reductase inhibitors.
Biology: this compound’s role as a dihydrofolate reductase inhibitor makes it valuable in studying cellular processes involving folate metabolism.
Comparison with Similar Compounds
Cycloguanil is structurally similar to other dihydrofolate reductase inhibitors, such as pyrimethamine and methotrexate. this compound is unique in its specific activity against the Plasmodium parasite. Similar compounds include:
Pyrimethamine: Another dihydrofolate reductase inhibitor used in antimalarial treatments.
Methotrexate: A dihydrofolate reductase inhibitor used in cancer therapy.
Properties
IUPAC Name |
1-(4-chlorophenyl)-6,6-dimethyl-1,3,5-triazine-2,4-diamine | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C11H14ClN5/c1-11(2)16-9(13)15-10(14)17(11)8-5-3-7(12)4-6-8/h3-6H,1-2H3,(H4,13,14,15,16) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
QMNFFXRFOJIOKZ-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1(N=C(N=C(N1C2=CC=C(C=C2)Cl)N)N)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C11H14ClN5 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
152-53-4 (hydrochloride) | |
| Record name | Cycloguanil | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000516212 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID9022867 | |
| Record name | Cycloguanil | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID9022867 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
251.71 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
516-21-2 | |
| Record name | Cycloguanil | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=516-21-2 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Cycloguanil | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000516212 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Cycloguanil | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB14763 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Cycloguanil | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID9022867 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | CYCLOGUANIL | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/26RM326WVN | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
A: Cycloguanil inhibits the enzyme dihydrofolate reductase (DHFR) in the malaria parasite Plasmodium falciparum. [, , , ] This enzyme is crucial for the parasite's folate metabolism, which in turn is essential for DNA synthesis and cell division. [, , ]
A: By blocking DHFR, this compound disrupts the synthesis of tetrahydrofolic acid, a coenzyme vital for the synthesis of purines and pyrimidines, the building blocks of DNA. [, ] This ultimately leads to the death of the parasite. [, ] Research has also shown that this compound can impact downstream signaling pathways, such as STAT3 transcriptional activity, further contributing to its antimalarial effects. []
A: While primarily known for its activity against malaria parasites, this compound also demonstrates inhibitory activity against Trypanosoma brucei PTR1 (TbPTR1), the enzyme pteridine reductase. [] This suggests potential as a dual PTR and DHFR inhibitor for treating human African trypanosomiasis. []
ANone: The provided research papers focus primarily on the biological activity and pharmacokinetic properties of this compound. Information regarding its material compatibility and stability under various conditions is not extensively discussed.
ANone: this compound itself doesn't possess catalytic properties. It functions as an enzyme inhibitor rather than a catalyst. It is primarily used for its antimalarial properties due to its inhibitory effect on DHFR.
A: Yes, computational techniques like molecular docking and quantitative structure-activity relationship (QSAR) studies have been employed. [, ] These methods help predict the binding affinities of this compound analogs with wild-type and mutant P. falciparum DHFR. [, ] Researchers have used programs like GOLD, FlexX, Glide, and Molegro to analyze and visualize interactions with the DHFR active site. []
A: Molecular docking studies suggest that P. falciparum DHFR exhibits stereoselectivity when binding to R and S enantiomers of this compound analogs. [] this compound analogs with alkyl chain substituents favor binding to the R enantiomer as it minimizes steric hindrance with the Phe58 residue in the DHFR active site. [] Conversely, analogs with phenol chain substituents prefer the S enantiomer, avoiding steric clashes with Leu46 and Met55. []
ANone: The provided scientific research papers primarily focus on characterizing the mechanism of action, resistance patterns, and structure-activity relationships of this compound and its analogs. They do not extensively cover aspects such as SHE regulations, detailed toxicology profiles, drug delivery strategies, environmental impact, or other points mentioned in questions 8-26.
A: Researchers frequently use in vitro drug susceptibility tests, often employing isotopic methods, to assess the efficacy of this compound against Plasmodium falciparum. [, , , , ] These tests involve culturing the parasite in the presence of varying concentrations of this compound and measuring its growth inhibition. [, , , , ] The concentration at which parasite growth is inhibited by 50% (IC50) is often used to compare the drug sensitivity of different parasite isolates. [, , , , ]
A: Yes, the composition of the culture medium can significantly influence the in vitro activity of this compound. [] Studies have demonstrated that standard RPMI medium or RPMI with low concentrations of folate and para-aminobenzoic acid leads to higher IC50 values for this compound compared to folate and para-aminobenzoic acid-free RPMI. [] These findings highlight the importance of carefully controlling for medium composition when performing in vitro drug susceptibility assays.
ANone: While the provided research focuses on specific aspects of this compound's activity and resistance, detailed toxicological data and long-term safety profiles are not extensively discussed.
ANone: The provided research papers predominantly concentrate on the mechanisms of action, resistance patterns, and structure-activity relationships of this compound and its analogs. Information regarding drug delivery, biomarkers, detailed analytical techniques, environmental impact, or other points mentioned in questions 13-26 is limited within these specific research papers.
A: this compound has a history dating back to the mid-20th century, emerging as a key player in antimalarial research. [] Initially, it was observed that the antimalarial activity of the prodrug chloroguanide hydrochloride was largely attributed to its dihydrotriazine metabolite, later identified as this compound. [] This led to the development of this compound pamoate, a poorly water-soluble salt form designed for intramuscular depot administration, to achieve prolonged antimalarial effects. []
A: Research on this compound demonstrates the synergy between various scientific disciplines, including parasitology, pharmacology, medicinal chemistry, and computational chemistry. [, ] Understanding the mechanism of action, resistance mechanisms, and structure-activity relationships through both in vitro and in vivo studies has been crucial in guiding the development of more effective antimalarial therapies. [, ] The identification of this compound as a potential inhibitor of Trypanosoma brucei PTR1 highlights the potential for cross-disciplinary applications in combating other parasitic diseases. []
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.









![5-methyl-2-[(2R)-6-methylhept-5-en-2-yl]phenol](/img/structure/B1669342.png)