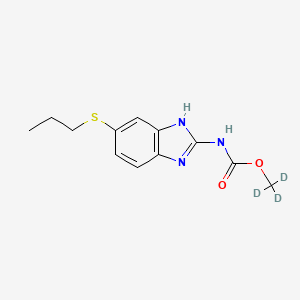
Albendazole-D3
Número de catálogo B1528130
Peso molecular: 268.35 g/mol
Clave InChI: HXHWSAZORRCQMX-BMSJAHLVSA-N
Atención: Solo para uso de investigación. No para uso humano o veterinario.
Patent
US08912225B2
Procedure details


SKOV-3 human cystadenocarcinoma cell line, obtained from the American Type Culture Collection (ATCC Accession No. HTB 77) were grown in McCoy's 5a medium with 1.5 mM L-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin, supplemented with 10% FCS. Cells were grown to confluence and harvested by trypsinization with 0.25 mg/ml trypsin/EDTA and suspended in the medium before plating. These were then seeded (2×105) on plastic 6-well Corning culture plates. Cultures were maintained in a 37° C. incubator in a humidified atmosphere of 95% O2/5% CO2. Twenty-four hours later, the medium was removed. Subconfluent cultures were washed three times with phosphate buffer followed by incubation for 6 hours with culture medium containing various concentrations of albendazole (0, 0.1, 0.25 and 1.0 μM) dissolved in 1% ethanol. After completion of the treatment period, medium from the wells were individually collected and analysed for the VEGF concentration using an enzyme-linked immunosorbant assay (ELISA) that detects soluble VEGF121 and VEGF165 isoforms (Quantikine R&D systems, Minneapolis, USA).
[Compound]
Name
5a
Quantity
0 (± 1) mol
Type
reactant
Reaction Step One



Name
streptomycin
Quantity
0 (± 1) mol
Type
reactant
Reaction Step Four



Name
Identifiers


|
REACTION_CXSMILES
|
N[C@H]([C:8]([OH:10])=[O:9])CCC(=O)N.CC1(C)[S:16][C@@H:15]2[C@H:17](NC(CC3C=CC=CC=3)=O)[C:18](=O)N2[C@H]1C([O-])=O.[K+].C[C@@H]1O[C@@H](O[C@H:42]2[C@H:47](O)[C@@H:46](O)[C@H:45](NC(N)=N)[C@@H:44](O)[C@@H:43]2[NH:55][C:56]([NH2:58])=[NH:57])[C@H](O[C@@H]2O[C@@H](CO)[C@H](O)[C@@H](O)[C@@H]2NC)[C@@]1(O)C=O.[CH2:75](N(CC(O)=O)CC(O)=O)CN(CC(O)=O)CC(O)=O.O=O>>[CH3:18][CH2:17][CH2:15][S:16][C:47]1[CH:46]=[CH:45][C:44]2[N:58]=[C:56]([NH:57][C:8]([O:10][CH3:75])=[O:9])[NH:55][C:43]=2[CH:42]=1 |f:1.2|
|
Inputs


Step One
[Compound]
|
Name
|
5a
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
|
Step Two
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
N[C@@H](CCC(N)=O)C(=O)O
|
Step Three
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
CC1([C@@H](N2[C@H](S1)[C@@H](C2=O)NC(=O)CC=3C=CC=CC3)C(=O)[O-])C.[K+]
|
Step Four
|
Name
|
streptomycin
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C[C@H]1[C@@]([C@H]([C@@H](O1)O[C@@H]2[C@H]([C@@H]([C@H]([C@@H]([C@H]2O)O)NC(=N)N)O)NC(=N)N)O[C@H]3[C@H]([C@@H]([C@H]([C@@H](O3)CO)O)O)NC)(C=O)O
|
Step Five
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(CN(CC(=O)O)CC(=O)O)N(CC(=O)O)CC(=O)O
|
Step Six
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
O=O
|
Conditions


Other
|
Conditions are dynamic
|
1
|
|
Details
|
See reaction.notes.procedure_details.
|
Workups


CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
SKOV-3 human cystadenocarcinoma cell line, obtained from the American Type Culture Collection (ATCC Accession No. HTB 77)
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
Twenty-four hours later, the medium was removed
|
WASH
|
Type
|
WASH
|
|
Details
|
Subconfluent cultures were washed three times with phosphate buffer
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
containing various
|
CONCENTRATION
|
Type
|
CONCENTRATION
|
|
Details
|
concentrations of albendazole (0, 0.1, 0.25 and 1.0 μM)
|
DISSOLUTION
|
Type
|
DISSOLUTION
|
|
Details
|
dissolved in 1% ethanol
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
After completion of the treatment period, medium from the wells were individually collected
|
CONCENTRATION
|
Type
|
CONCENTRATION
|
|
Details
|
analysed for the VEGF concentration
|
Outcomes


Product
Source


|
Source
|
Open Reaction Database (ORD) |
|
Description
|
The Open Reaction Database (ORD) is an open-access schema and infrastructure for structuring and sharing organic reaction data, including a centralized data repository. The ORD schema supports conventional and emerging technologies, from benchtop reactions to automated high-throughput experiments and flow chemistry. Our vision is that a consistent data representation and infrastructure to support data sharing will enable downstream applications that will greatly improve the state of the art with respect to computer-aided synthesis planning, reaction prediction, and other predictive chemistry tasks. |
Patent
US08912225B2
Procedure details


SKOV-3 human cystadenocarcinoma cell line, obtained from the American Type Culture Collection (ATCC Accession No. HTB 77) were grown in McCoy's 5a medium with 1.5 mM L-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin, supplemented with 10% FCS. Cells were grown to confluence and harvested by trypsinization with 0.25 mg/ml trypsin/EDTA and suspended in the medium before plating. These were then seeded (2×105) on plastic 6-well Corning culture plates. Cultures were maintained in a 37° C. incubator in a humidified atmosphere of 95% O2/5% CO2. Twenty-four hours later, the medium was removed. Subconfluent cultures were washed three times with phosphate buffer followed by incubation for 6 hours with culture medium containing various concentrations of albendazole (0, 0.1, 0.25 and 1.0 μM) dissolved in 1% ethanol. After completion of the treatment period, medium from the wells were individually collected and analysed for the VEGF concentration using an enzyme-linked immunosorbant assay (ELISA) that detects soluble VEGF121 and VEGF165 isoforms (Quantikine R&D systems, Minneapolis, USA).
[Compound]
Name
5a
Quantity
0 (± 1) mol
Type
reactant
Reaction Step One



Name
streptomycin
Quantity
0 (± 1) mol
Type
reactant
Reaction Step Four



Name
Identifiers


|
REACTION_CXSMILES
|
N[C@H]([C:8]([OH:10])=[O:9])CCC(=O)N.CC1(C)[S:16][C@@H:15]2[C@H:17](NC(CC3C=CC=CC=3)=O)[C:18](=O)N2[C@H]1C([O-])=O.[K+].C[C@@H]1O[C@@H](O[C@H:42]2[C@H:47](O)[C@@H:46](O)[C@H:45](NC(N)=N)[C@@H:44](O)[C@@H:43]2[NH:55][C:56]([NH2:58])=[NH:57])[C@H](O[C@@H]2O[C@@H](CO)[C@H](O)[C@@H](O)[C@@H]2NC)[C@@]1(O)C=O.[CH2:75](N(CC(O)=O)CC(O)=O)CN(CC(O)=O)CC(O)=O.O=O>>[CH3:18][CH2:17][CH2:15][S:16][C:47]1[CH:46]=[CH:45][C:44]2[N:58]=[C:56]([NH:57][C:8]([O:10][CH3:75])=[O:9])[NH:55][C:43]=2[CH:42]=1 |f:1.2|
|
Inputs


Step One
[Compound]
|
Name
|
5a
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
|
Step Two
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
N[C@@H](CCC(N)=O)C(=O)O
|
Step Three
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
CC1([C@@H](N2[C@H](S1)[C@@H](C2=O)NC(=O)CC=3C=CC=CC3)C(=O)[O-])C.[K+]
|
Step Four
|
Name
|
streptomycin
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C[C@H]1[C@@]([C@H]([C@@H](O1)O[C@@H]2[C@H]([C@@H]([C@H]([C@@H]([C@H]2O)O)NC(=N)N)O)NC(=N)N)O[C@H]3[C@H]([C@@H]([C@H]([C@@H](O3)CO)O)O)NC)(C=O)O
|
Step Five
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(CN(CC(=O)O)CC(=O)O)N(CC(=O)O)CC(=O)O
|
Step Six
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
O=O
|
Conditions


Other
|
Conditions are dynamic
|
1
|
|
Details
|
See reaction.notes.procedure_details.
|
Workups


CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
SKOV-3 human cystadenocarcinoma cell line, obtained from the American Type Culture Collection (ATCC Accession No. HTB 77)
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
Twenty-four hours later, the medium was removed
|
WASH
|
Type
|
WASH
|
|
Details
|
Subconfluent cultures were washed three times with phosphate buffer
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
containing various
|
CONCENTRATION
|
Type
|
CONCENTRATION
|
|
Details
|
concentrations of albendazole (0, 0.1, 0.25 and 1.0 μM)
|
DISSOLUTION
|
Type
|
DISSOLUTION
|
|
Details
|
dissolved in 1% ethanol
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
After completion of the treatment period, medium from the wells were individually collected
|
CONCENTRATION
|
Type
|
CONCENTRATION
|
|
Details
|
analysed for the VEGF concentration
|
Outcomes


Product
Source


|
Source
|
Open Reaction Database (ORD) |
|
Description
|
The Open Reaction Database (ORD) is an open-access schema and infrastructure for structuring and sharing organic reaction data, including a centralized data repository. The ORD schema supports conventional and emerging technologies, from benchtop reactions to automated high-throughput experiments and flow chemistry. Our vision is that a consistent data representation and infrastructure to support data sharing will enable downstream applications that will greatly improve the state of the art with respect to computer-aided synthesis planning, reaction prediction, and other predictive chemistry tasks. |
Patent
US08912225B2
Procedure details


SKOV-3 human cystadenocarcinoma cell line, obtained from the American Type Culture Collection (ATCC Accession No. HTB 77) were grown in McCoy's 5a medium with 1.5 mM L-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin, supplemented with 10% FCS. Cells were grown to confluence and harvested by trypsinization with 0.25 mg/ml trypsin/EDTA and suspended in the medium before plating. These were then seeded (2×105) on plastic 6-well Corning culture plates. Cultures were maintained in a 37° C. incubator in a humidified atmosphere of 95% O2/5% CO2. Twenty-four hours later, the medium was removed. Subconfluent cultures were washed three times with phosphate buffer followed by incubation for 6 hours with culture medium containing various concentrations of albendazole (0, 0.1, 0.25 and 1.0 μM) dissolved in 1% ethanol. After completion of the treatment period, medium from the wells were individually collected and analysed for the VEGF concentration using an enzyme-linked immunosorbant assay (ELISA) that detects soluble VEGF121 and VEGF165 isoforms (Quantikine R&D systems, Minneapolis, USA).
[Compound]
Name
5a
Quantity
0 (± 1) mol
Type
reactant
Reaction Step One



Name
streptomycin
Quantity
0 (± 1) mol
Type
reactant
Reaction Step Four



Name
Identifiers


|
REACTION_CXSMILES
|
N[C@H]([C:8]([OH:10])=[O:9])CCC(=O)N.CC1(C)[S:16][C@@H:15]2[C@H:17](NC(CC3C=CC=CC=3)=O)[C:18](=O)N2[C@H]1C([O-])=O.[K+].C[C@@H]1O[C@@H](O[C@H:42]2[C@H:47](O)[C@@H:46](O)[C@H:45](NC(N)=N)[C@@H:44](O)[C@@H:43]2[NH:55][C:56]([NH2:58])=[NH:57])[C@H](O[C@@H]2O[C@@H](CO)[C@H](O)[C@@H](O)[C@@H]2NC)[C@@]1(O)C=O.[CH2:75](N(CC(O)=O)CC(O)=O)CN(CC(O)=O)CC(O)=O.O=O>>[CH3:18][CH2:17][CH2:15][S:16][C:47]1[CH:46]=[CH:45][C:44]2[N:58]=[C:56]([NH:57][C:8]([O:10][CH3:75])=[O:9])[NH:55][C:43]=2[CH:42]=1 |f:1.2|
|
Inputs


Step One
[Compound]
|
Name
|
5a
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
|
Step Two
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
N[C@@H](CCC(N)=O)C(=O)O
|
Step Three
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
CC1([C@@H](N2[C@H](S1)[C@@H](C2=O)NC(=O)CC=3C=CC=CC3)C(=O)[O-])C.[K+]
|
Step Four
|
Name
|
streptomycin
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C[C@H]1[C@@]([C@H]([C@@H](O1)O[C@@H]2[C@H]([C@@H]([C@H]([C@@H]([C@H]2O)O)NC(=N)N)O)NC(=N)N)O[C@H]3[C@H]([C@@H]([C@H]([C@@H](O3)CO)O)O)NC)(C=O)O
|
Step Five
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(CN(CC(=O)O)CC(=O)O)N(CC(=O)O)CC(=O)O
|
Step Six
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
O=O
|
Conditions


Other
|
Conditions are dynamic
|
1
|
|
Details
|
See reaction.notes.procedure_details.
|
Workups


CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
SKOV-3 human cystadenocarcinoma cell line, obtained from the American Type Culture Collection (ATCC Accession No. HTB 77)
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
Twenty-four hours later, the medium was removed
|
WASH
|
Type
|
WASH
|
|
Details
|
Subconfluent cultures were washed three times with phosphate buffer
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
containing various
|
CONCENTRATION
|
Type
|
CONCENTRATION
|
|
Details
|
concentrations of albendazole (0, 0.1, 0.25 and 1.0 μM)
|
DISSOLUTION
|
Type
|
DISSOLUTION
|
|
Details
|
dissolved in 1% ethanol
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
After completion of the treatment period, medium from the wells were individually collected
|
CONCENTRATION
|
Type
|
CONCENTRATION
|
|
Details
|
analysed for the VEGF concentration
|
Outcomes


Product
Source


|
Source
|
Open Reaction Database (ORD) |
|
Description
|
The Open Reaction Database (ORD) is an open-access schema and infrastructure for structuring and sharing organic reaction data, including a centralized data repository. The ORD schema supports conventional and emerging technologies, from benchtop reactions to automated high-throughput experiments and flow chemistry. Our vision is that a consistent data representation and infrastructure to support data sharing will enable downstream applications that will greatly improve the state of the art with respect to computer-aided synthesis planning, reaction prediction, and other predictive chemistry tasks. |
