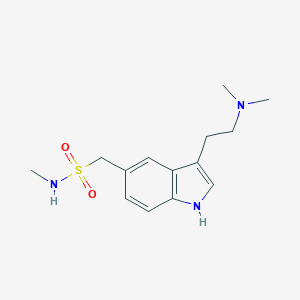
Sumatriptan
Número de catálogo B127528
Peso molecular: 295.40 g/mol
Clave InChI: KQKPFRSPSRPDEB-UHFFFAOYSA-N
Atención: Solo para uso de investigación. No para uso humano o veterinario.
Patent
US06255502B1
Procedure details


Sumatriptan caprate and hydroxypropyl-beta cyclodextrin (HPB) were complexed by the kneading method. Sumatriptan caprate (1.254 g) and HPB (3.748 g) were blended together. Water (4.5 mL) was added and the mixture ground together in a mortar with a pestle to form a uniform paste. Grinding was continued for 30 minutes. The paste was then dried in a vacuum oven (40° C.; 0 bar) for 48 hours. The solid mass was broken up, passed through a 60 mesh screen and returned to the vacuum oven (40° C.; 0 bar) for 12 hours in order to ensure uniform drying of the complex. Analysis by HPLC for sumatriptan base content, and Karl Fischer for moisture content gave the following results: % sumatriptan base was 16.40% and the moisture content was 3.45%. The complex was characterised by DSC, FT-IR and XRD.
Name
Sumatriptan caprate
Quantity
0 (± 1) mol
Type
reactant
Reaction Step One

Name
hydroxypropyl-beta cyclodextrin
Quantity
0 (± 1) mol
Type
reactant
Reaction Step Two

Name
Sumatriptan caprate
Quantity
1.254 g
Type
reactant
Reaction Step Three

Name
HPB
Quantity
3.748 g
Type
reactant
Reaction Step Four


Name
Identifiers


|
REACTION_CXSMILES
|
[CH3:1][NH:2][S:3]([CH2:6][C:7]1[CH:8]=[CH:9][C:10]2[NH:15][CH:14]=[C:13]([CH2:16][CH2:17][N:18]([CH3:20])[CH3:19])[C:11]=2[CH:12]=1)(=[O:5])=[O:4].[O-]C(CCCCCCCCC)=O.CC(O)COC[C@H]1O[C@@H]2O[C@H]3[C@H](O)[C@@H](O)[C@@H](O[C@H]4[C@H](O)[C@@H](O)[C@@H](O[C@H]5[C@H](O)[C@@H](O)[C@@H](O[C@H]6[C@H](O)[C@@H](O)[C@@H](O[C@H]7[C@H](O)[C@@H](O)[C@@H](O[C@H]8[C@H](O)[C@@H](O)[C@@H](O[C@H]1[C@H](O)[C@H]2O)O[C@@H]8COCC(O)C)O[C@@H]7COCC(O)C)O[C@@H]6COCC(O)C)O[C@@H]5COCC(O)C)O[C@@H]4COCC(O)C)O[C@@H]3COCC(O)C>O>[CH3:1][NH:2][S:3]([CH2:6][C:7]1[CH:8]=[CH:9][C:10]2[NH:15][CH:14]=[C:13]([CH2:16][CH2:17][N:18]([CH3:20])[CH3:19])[C:11]=2[CH:12]=1)(=[O:5])=[O:4] |f:0.1|
|
Inputs


Step One
|
Name
|
Sumatriptan caprate
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
CNS(=O)(=O)CC=1C=CC2=C(C1)C(=CN2)CCN(C)C.[O-]C(=O)CCCCCCCCC
|
Step Two
|
Name
|
hydroxypropyl-beta cyclodextrin
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
CC(COC[C@@H]1[C@@H]2[C@@H]([C@H]([C@H](O1)O[C@@H]3[C@H](O[C@@H]([C@@H]([C@H]3O)O)O[C@@H]4[C@H](O[C@@H]([C@@H]([C@H]4O)O)O[C@@H]5[C@H](O[C@@H]([C@@H]([C@H]5O)O)O[C@@H]6[C@H](O[C@@H]([C@@H]([C@H]6O)O)O[C@@H]7[C@H](O[C@@H]([C@@H]([C@H]7O)O)O[C@@H]8[C@H](O[C@H](O2)[C@@H]([C@H]8O)O)COCC(C)O)COCC(C)O)COCC(C)O)COCC(C)O)COCC(C)O)COCC(C)O)O)O)O
|
Step Three
|
Name
|
Sumatriptan caprate
|
|
Quantity
|
1.254 g
|
|
Type
|
reactant
|
|
Smiles
|
CNS(=O)(=O)CC=1C=CC2=C(C1)C(=CN2)CCN(C)C.[O-]C(=O)CCCCCCCCC
|
Step Four
|
Name
|
HPB
|
|
Quantity
|
3.748 g
|
|
Type
|
reactant
|
|
Smiles
|
CC(COC[C@@H]1[C@@H]2[C@@H]([C@H]([C@H](O1)O[C@@H]3[C@H](O[C@@H]([C@@H]([C@H]3O)O)O[C@@H]4[C@H](O[C@@H]([C@@H]([C@H]4O)O)O[C@@H]5[C@H](O[C@@H]([C@@H]([C@H]5O)O)O[C@@H]6[C@H](O[C@@H]([C@@H]([C@H]6O)O)O[C@@H]7[C@H](O[C@@H]([C@@H]([C@H]7O)O)O[C@@H]8[C@H](O[C@H](O2)[C@@H]([C@H]8O)O)COCC(C)O)COCC(C)O)COCC(C)O)COCC(C)O)COCC(C)O)COCC(C)O)O)O)O
|
Step Five
|
Name
|
|
|
Quantity
|
4.5 mL
|
|
Type
|
solvent
|
|
Smiles
|
O
|
Conditions


Other
|
Conditions are dynamic
|
1
|
|
Details
|
See reaction.notes.procedure_details.
|
Workups


CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
the mixture ground together in a mortar with a pestle to form a uniform paste
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
Grinding
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
The paste was then dried in a vacuum oven (40° C.; 0 bar) for 48 hours
|
|
Duration
|
48 h
|
WAIT
|
Type
|
WAIT
|
|
Details
|
returned to the vacuum oven (40° C.; 0 bar) for 12 hours in order
|
|
Duration
|
12 h
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
to ensure uniform drying of the complex
|
Outcomes


Product
Details
Reaction Time |
30 min |
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
CNS(=O)(=O)CC=1C=CC2=C(C1)C(=CN2)CCN(C)C
|
Source


|
Source
|
Open Reaction Database (ORD) |
|
Description
|
The Open Reaction Database (ORD) is an open-access schema and infrastructure for structuring and sharing organic reaction data, including a centralized data repository. The ORD schema supports conventional and emerging technologies, from benchtop reactions to automated high-throughput experiments and flow chemistry. Our vision is that a consistent data representation and infrastructure to support data sharing will enable downstream applications that will greatly improve the state of the art with respect to computer-aided synthesis planning, reaction prediction, and other predictive chemistry tasks. |
