Ledipasvir
Vue d'ensemble
Description
Le lédipasvir est un antiviral à action directe principalement utilisé pour le traitement des infections chroniques par le virus de l'hépatite C (VHC). Il a été développé par Gilead Sciences et est couramment utilisé en association avec le sofosbuvir sous le nom de marque Harvoni. Le lédipasvir cible la protéine non structurale 5A (NS5A) du virus de l'hépatite C, qui est essentielle à la réplication et à l'assemblage viral .
Applications De Recherche Scientifique
Ledipasvir has significant applications in scientific research, particularly in the fields of chemistry, biology, medicine, and industry:
Chemistry: this compound serves as a model compound for studying antiviral agents and their synthesis.
Biology: It is used to study the replication mechanisms of the hepatitis C virus and the role of NS5A in viral assembly.
Medicine: This compound, in combination with sofosbuvir, is used to treat chronic hepatitis C infections, achieving high cure rates and minimal side effects
Industry: The production and formulation of this compound contribute to the pharmaceutical industry’s efforts to develop effective antiviral therapies
Mécanisme D'action
Target of Action
Ledipasvir primarily targets the Non-Structural Protein 5A (NS5A) of the Hepatitis C Virus (HCV) . NS5A is a multifunctional protein that plays crucial roles in viral RNA replication and the assembly of HCV virions .
Mode of Action
This compound acts as an inhibitor of the HCV NS5A protein . This inhibition disrupts the normal functioning of the NS5A protein, thereby impeding the life cycle of the HCV .
Biochemical Pathways
The primary biochemical pathway affected by this compound is the replication of the HCV RNA genome . By inhibiting the NS5A protein, this compound disrupts the replication of the viral RNA, which in turn affects the assembly of new HCV virions . This leads to a decrease in the overall viral load within the host.
Pharmacokinetics
This compound exhibits a bioavailability of 76% . It reaches maximum plasma concentrations 4 to 4.5 hours after ingestion . This compound is more than 98% protein-bound and is predominantly eliminated fecally, with minimal metabolism in the liver . The elimination half-life of this compound is approximately 47 hours .
Result of Action
The inhibition of the NS5A protein by this compound results in a significant reduction in HCV RNA replication . This leads to a decrease in the production of new HCV virions, thereby reducing the overall viral load within the host. The ultimate goal of this compound treatment is to achieve a sustained virologic response (SVR), which is defined as the absence of detectable HCV RNA 12 weeks after the completion of therapy . Achieving SVR is associated with long-term health benefits, including reduced liver-related damage, improved quality of life, reduced incidence of Hepatocellular Carcinoma, and reduced all-cause mortality .
Action Environment
Analyse Biochimique
Biochemical Properties
Ledipasvir interacts with the NS5A protein, a phosphoprotein that plays an essential role in the replication of the hepatitis C virus . By binding to this protein, this compound disrupts the replication process of the virus .
Cellular Effects
This compound has a profound effect on cells infected with the hepatitis C virus. It inhibits the replication of the virus, thereby reducing the viral load within the cells . This can lead to a decrease in the severity of the disease and potentially to a complete cure .
Molecular Mechanism
The molecular mechanism of this compound involves its interaction with the NS5A protein. This compound binds to this protein, preventing it from assisting in the replication of the viral RNA . This stops the virus from multiplying and spreading to other cells .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of this compound have been observed to be both rapid and long-lasting . The drug quickly reduces the viral load in cells, and this effect can be sustained over a long period, leading to a sustained virological response .
Dosage Effects in Animal Models
While specific studies on dosage effects in animal models were not found in the search results, clinical studies have shown that this compound, in combination with other antiviral drugs, is effective in treating hepatitis C in humans .
Metabolic Pathways
It is known that the drug works by interfering with the life cycle of the hepatitis C virus, specifically by inhibiting the NS5A protein .
Transport and Distribution
Given its effectiveness in reducing viral load, it can be inferred that the drug is able to reach the sites of viral replication within the cells .
Subcellular Localization
Given that it targets the NS5A protein, which is involved in the replication of the hepatitis C virus, it is likely that the drug localizes to the sites within the cell where this process takes place .
Méthodes De Préparation
Voies de synthèse et conditions réactionnelles
La synthèse du lédipasvir implique plusieurs étapes, y compris la préparation d'intermédiaires clés. Une méthode implique la préparation d'un intermédiaire de haute pureté, l'acide (1R, 3S, 4S)-N-tertbutyloxycarbonyl-2-azabicyclo[2.2.1]heptane-3-carboxylique, par hydrolyse enzymatique . Une autre méthode implique des processus de cyclopropanation et de fluoration en fin de synthèse, qui offrent une voie nouvelle et efficace pour la préparation du lédipasvir avec un rendement total de 20% sur huit étapes linéaires .
Méthodes de production industrielle
La production industrielle du lédipasvir est axée sur l'optimisation du rendement, de la pureté et de la rentabilité. Le processus implique généralement l'utilisation d'intermédiaires de haute pureté et de conditions de réaction respectueuses de l'environnement. Les méthodes sont conçues pour être évolutives pour une production à grande échelle, assurant une grande sélectivité et des coûts de production réduits .
Analyse Des Réactions Chimiques
Types de réactions
Le lédipasvir subit diverses réactions chimiques, notamment :
Réduction : Les réactions de réduction sont moins courantes pour le lédipasvir.
Substitution : Les réactions de substitution, en particulier celles impliquant des atomes d'halogène, sont plus pertinentes dans la synthèse du lédipasvir
Réactifs et conditions courants
Les réactifs courants utilisés dans la synthèse et les réactions du lédipasvir comprennent l'acétonitrile, l'acide acétique et l'éther isopropylique. Les conditions réactionnelles impliquent souvent des températures élevées et des environnements contrôlés pour garantir un rendement et une pureté élevés .
Principaux produits formés
Le principal produit formé par les réactions impliquant le lédipasvir est l'ingrédient pharmaceutique actif final utilisé dans le traitement de l'hépatite C. Les autres intermédiaires et sous-produits sont généralement éliminés par des processus de purification .
Applications de recherche scientifique
Le lédipasvir a des applications significatives dans la recherche scientifique, en particulier dans les domaines de la chimie, de la biologie, de la médecine et de l'industrie :
Chimie : Le lédipasvir sert de composé modèle pour étudier les agents antiviraux et leur synthèse.
Biologie : Il est utilisé pour étudier les mécanismes de réplication du virus de l'hépatite C et le rôle de la NS5A dans l'assemblage viral.
Médecine : Le lédipasvir, associé au sofosbuvir, est utilisé pour traiter les infections chroniques par le virus de l'hépatite C, atteignant des taux de guérison élevés et des effets secondaires minimes
Industrie : La production et la formulation du lédipasvir contribuent aux efforts de l'industrie pharmaceutique pour développer des thérapies antivirales efficaces
Mécanisme d'action
Le lédipasvir inhibe la protéine non structurale 5A (NS5A) du virus de l'hépatite C, qui est essentielle à la réplication de l'ARN viral et à l'assemblage des virions du VHC. En empêchant l'hyperphosphorylation de la NS5A, le lédipasvir perturbe la production de protéines virales, inhibant ainsi la réplication et l'assemblage viral .
Comparaison Avec Des Composés Similaires
Composés similaires
Sofosbuvir : Un autre antiviral à action directe utilisé en association avec le lédipasvir. Il inhibe la polymérase NS5B du VHC.
Daclatasvir : Un inhibiteur de la NS5A similaire au lédipasvir mais avec des propriétés pharmacocinétiques différentes.
Ombitasvir : Un autre inhibiteur de la NS5A utilisé en association avec d'autres agents antiviraux pour le traitement du VHC
Unicité du lédipasvir
Le lédipasvir est unique en raison de sa forte puissance contre plusieurs génotypes du VHC et de sa capacité à atteindre des taux de réponse virologique durable (RVS) de plus de 95 % lorsqu'il est utilisé en association avec le sofosbuvir. Sa longue demi-vie et ses effets secondaires minimes en font un choix privilégié pour le traitement du VHC .
Propriétés
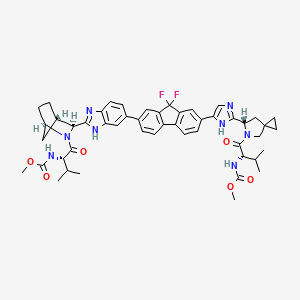
IUPAC Name |
methyl N-[(2S)-1-[(6S)-6-[5-[9,9-difluoro-7-[2-[(1R,3S,4S)-2-[(2S)-2-(methoxycarbonylamino)-3-methylbutanoyl]-2-azabicyclo[2.2.1]heptan-3-yl]-3H-benzimidazol-5-yl]fluoren-2-yl]-1H-imidazol-2-yl]-5-azaspiro[2.4]heptan-5-yl]-3-methyl-1-oxobutan-2-yl]carbamate | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C49H54F2N8O6/c1-24(2)39(56-46(62)64-5)44(60)58-23-48(15-16-48)21-38(58)42-52-22-37(55-42)28-9-13-32-31-12-8-26(18-33(31)49(50,51)34(32)19-28)27-10-14-35-36(20-27)54-43(53-35)41-29-7-11-30(17-29)59(41)45(61)40(25(3)4)57-47(63)65-6/h8-10,12-14,18-20,22,24-25,29-30,38-41H,7,11,15-17,21,23H2,1-6H3,(H,52,55)(H,53,54)(H,56,62)(H,57,63)/t29-,30+,38-,39-,40-,41-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
VRTWBAAJJOHBQU-KMWAZVGDSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)C(C(=O)N1CC2(CC2)CC1C3=NC=C(N3)C4=CC5=C(C=C4)C6=C(C5(F)F)C=C(C=C6)C7=CC8=C(C=C7)N=C(N8)C9C1CCC(C1)N9C(=O)C(C(C)C)NC(=O)OC)NC(=O)OC | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC(C)[C@@H](C(=O)N1CC2(CC2)C[C@H]1C3=NC=C(N3)C4=CC5=C(C=C4)C6=C(C5(F)F)C=C(C=C6)C7=CC8=C(C=C7)N=C(N8)[C@@H]9[C@H]1CC[C@H](C1)N9C(=O)[C@H](C(C)C)NC(=O)OC)NC(=O)OC | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C49H54F2N8O6 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID90154829 | |
| Record name | Ledipasvir | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID90154829 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
889.0 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Mechanism of Action |
Ledipasvir is an inhibitor of the Hepatitis C Virus (HCV) NS5A protein required for viral RNA replication and assembly of HCV virions. Although its exact mechanism of action is unknown, it is postulated to prevent hyperphosphorylation of NS5A which is required for viral production. | |
| Record name | Ledipasvir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09027 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
1256388-51-8 | |
| Record name | Ledipasvir | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=1256388-51-8 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Ledipasvir [USAN:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=1256388518 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Ledipasvir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09027 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Ledipasvir | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID90154829 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Methyl[(2S)-1-{(6S)-6-[5-(9,9-difluoro-7-{2-[(1R,3S,4S)-2-{(2S)-2-[(methoxycarbonyl)amino]-3-methylbutanoyl}-2-azabicyclo[2.2.1]hept-3-yl]-1H-benzimidazol-6-yl}-9H-fluoren-2-yl)-1H-imidazol-2-yl]-5-azaspiro[2.4]hept-5-yl}-3-methyl-1-oxobutan-2-yl]carbamate | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | LEDIPASVIR | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/013TE6E4WV | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Synthesis routes and methods I
Procedure details












Synthesis routes and methods II
Procedure details












Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.
![Thioacetic acid S-[(S)-6-[[(R)-5-oxopyrrolidine-2-ylcarbonyl]amino]-6-(phenylcarbamoyl)hexyl] ester](/img/structure/B612170.png)