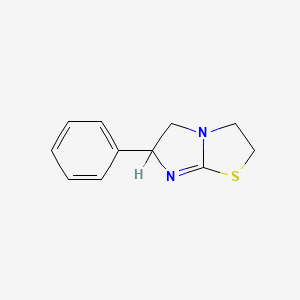
Tetramisole
概要
説明
テトラミゾールは、レバミゾールとそのエナンチオマーであるデキサミゾールのラセミ体混合物です。 組織非特異的アルカリホスファターゼの特異的阻害剤であり、獣医学では回虫症や鉤虫などの寄生虫感染症の治療に使用されます 。 テトラミゾールは免疫刺激作用も有しており、この文脈ではより長く使用されてきました .
準備方法
化学反応の分析
テトラミゾールは、次のようなさまざまな化学反応を起こします。
酸化: テトラミゾールは特定の条件下で酸化される可能性がありますが、詳細な酸化経路はあまり報告されていません。
還元: 還元反応は、通常、テトラミゾールとは関連付けられていません。
これらの反応で使用される一般的な試薬には、炭酸ナトリウム、塩酸、およびN,N-ジメチルホルムアミドなどの有機溶媒が含まれます 。これらの反応から生成される主要な生成物は、使用される特定の条件と試薬によって異なります。
科学研究への応用
テトラミゾールは、幅広い科学研究への応用があります。
科学的研究の応用
Tetramisole has a wide range of scientific research applications:
Chemistry: This compound is used as a reagent in various chemical reactions and as an inhibitor in enzymatic studies.
Medicine: This compound has been used as an anthelmintic to treat worm infections and as an immunostimulant.
Industry: This compound is used in veterinary medicine to treat parasitic infections in livestock and pets.
作用機序
類似化合物との比較
テトラミゾールは、純粋なL-異性体であるレバミゾールと比較されることがよくあります。 レバミゾールは、駆虫剤および免疫刺激剤として同様の特性を持っていますが、ヒト医学でより一般的に使用されています 。 その他の類似の化合物には、ブタミゾールがありますが、用途が限られているため、ほとんど使用されなくなりました 。 テトラミゾールの駆虫剤と免疫刺激剤の独特な組み合わせは、組織非特異的アルカリホスファターゼを阻害する能力とともに、同クラスの他の化合物とは異なります .
特性
IUPAC Name |
6-phenyl-2,3,5,6-tetrahydroimidazo[2,1-b][1,3]thiazole | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C11H12N2S/c1-2-4-9(5-3-1)10-8-13-6-7-14-11(13)12-10/h1-5,10H,6-8H2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
HLFSDGLLUJUHTE-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1CSC2=NC(CN21)C3=CC=CC=C3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C11H12N2S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID20860143 | |
| Record name | Imidazo[2,1-b]thiazole, 2,3,5,6-tetrahydro-6-phenyl- | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID20860143 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
204.29 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
5036-02-2 | |
| Record name | (±)-Tetramisole | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=5036-02-2 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Tetramisole [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0005036022 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Tetramisole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB16561 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Imidazo[2,1-b]thiazole, 2,3,5,6-tetrahydro-6-phenyl- | |
| Source | EPA Chemicals under the TSCA | |
| URL | https://www.epa.gov/chemicals-under-tsca | |
| Description | EPA Chemicals under the Toxic Substances Control Act (TSCA) collection contains information on chemicals and their regulations under TSCA, including non-confidential content from the TSCA Chemical Substance Inventory and Chemical Data Reporting. | |
| Record name | Imidazo[2,1-b]thiazole, 2,3,5,6-tetrahydro-6-phenyl- | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID20860143 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Tetramisole | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.023.391 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | TETRAMISOLE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/C8M7RFE4NO | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Synthesis routes and methods I
Procedure details





Synthesis routes and methods II
Procedure details





Synthesis routes and methods III
Procedure details





Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary mechanism of action of tetramisole?
A1: this compound acts as a potent inhibitor of alkaline phosphatase, particularly certain isoenzymes found in organisms like the parasitic worm Ascaris suum []. While its precise mechanism in parasite paralysis remains unclear, studies suggest it causes sustained muscle contraction and membrane depolarization, potentially through interaction with cholinergic systems [, ].
Q2: How does this compound's structure relate to its inhibition of alkaline phosphatase?
A2: Research indicates that the presence of both the thiazolidine and dihydroimidazole rings in the this compound structure is crucial for its inhibitory activity against alkaline phosphatase [, ]. Replacing the thiazole ring system significantly reduces its potency [].
Q3: Does the stereochemistry of this compound influence its alkaline phosphatase inhibition?
A3: Yes, the (-) isomer of this compound, also known as levamisole, exhibits significantly greater inhibition of specific alkaline phosphatase isoenzymes compared to the (+) isomer, dexamisole [, ].
Q4: How does this compound affect cartilage calcification?
A4: Levamisole, the (-) isomer, has been shown to prevent in vitro cartilage calcification in rachitic rat models by inhibiting alkaline phosphatase activity within cartilage []. This suggests a close association between ALP activity and the calcification process.
Q5: Does this compound affect interferon production?
A5: Studies show that this compound can modulate interferon-beta (INF-β) production in a dose-dependent manner. High concentrations suppress INF-β synthesis in mouse peritoneal cells, while lower concentrations might increase synthesis when baseline production is low [].
Q6: How does this compound impact amino acid uptake in parasites?
A6: In Ascaridia galli (chicken roundworm), both racemic this compound and levamisole inhibit the uptake of essential amino acids like aspartic acid, alanine, and leucine, ultimately affecting protein synthesis and other crucial metabolic processes [].
Q7: What is the molecular formula and weight of this compound?
A7: The molecular formula of this compound is C11H12N2S, and its molecular weight is 204.29 g/mol.
Q8: Are there specific challenges in formulating this compound?
A8: Research highlights the importance of specific formulation strategies for this compound. For instance, loading this compound into a zeolite matrix allowed for slow drug release, enhancing its efficacy against Nippostrongylus brasiliensis in rats compared to administering this compound alone [].
Q9: Does this compound exhibit any catalytic properties?
A9: While primarily known for its anthelmintic and immunomodulatory properties, the research provided does not elaborate on any inherent catalytic properties of this compound.
Q10: Have computational methods been used in this compound research?
A10: The provided research papers primarily focus on experimental studies and do not delve into specific applications of computational chemistry or modeling regarding this compound.
Q11: How do structural modifications impact this compound's activity?
A11: Studies demonstrate that the thiazolidine and dihydroimidazole rings are essential for the anthelmintic activity of this compound [, ]. Modifications like substituting the phenyl ring with naphthyl or thienyl groups, or introducing halogen, methyl, or nitro groups, can significantly alter its potency and selectivity against different alkaline phosphatase isoenzymes [, ].
Q12: Are there specific formulation challenges for this compound?
A12: While the provided research doesn't highlight specific formulation challenges, one study successfully loaded this compound into a zeolite matrix for slow drug release, enhancing its efficacy against Nippostrongylus brasiliensis in rats []. This suggests that optimizing drug delivery can improve this compound's effectiveness.
Q13: Does the research address SHE regulations regarding this compound?
A13: The provided research articles primarily focus on the pharmacological and biochemical aspects of this compound. Specific information regarding SHE regulations is not discussed in these papers.
Q14: How is this compound metabolized in the body?
A14: While detailed metabolic pathways are not extensively discussed, research indicates potential for stereoselective metabolism between levamisole and dexamisole. A study observed significantly longer elimination half-lives for dexamisole compared to levamisole in human subjects, suggesting differences in their metabolic fates [].
Q15: Are there differences in the pharmacokinetics of levamisole and dexamisole?
A15: Yes, studies reveal that dexamisole exhibits a longer elimination half-life compared to levamisole, suggesting stereoselective pharmacokinetic profiles for these enantiomers []. This difference may lead to varying durations of action and potential for adverse effects.
Q16: How is this compound's efficacy against parasites evaluated?
A16: Researchers utilize various methods to assess this compound's efficacy, including:
- Fecal Egg Count Reduction Test (FECRT): This test measures the reduction in parasite egg counts in animal feces after treatment, indicating the drug's effectiveness in reducing worm burden [, , , , ].
- Controlled Anthelmintic Efficacy Test: This involves comparing worm counts in treated animals with untreated controls to determine the percentage of parasite reduction [].
- In Vitro Egg Hatch Assay: This method assesses the drug's effect on parasite egg hatching, providing insights into its efficacy against early developmental stages [].
Q17: Has this compound shown efficacy in treating specific parasitic infections?
A17: Research indicates that this compound effectively treats infections caused by various parasitic nematodes, including:
- Ascaridia galli: this compound effectively eliminates both adult and immature stages of this chicken roundworm [, , , , ].
- Heterakis gallinarum: this compound demonstrates efficacy against this cecal worm in chickens [, , ].
- Capillaria obsignata: Studies show varying degrees of efficacy against this chicken intestinal worm depending on the dose and route of administration [].
- Dictyocaulus viviparus: this compound effectively targets lungworms in calves, particularly those older than 14 days [].
- Nippostrongylus brasiliensis: Formulating this compound for slow release enhances its efficacy against this rat nematode [].
Q18: What is the evidence for this compound's immunomodulatory activity?
A18: Research highlights this compound's potential to enhance immune responses. For instance, it significantly improved the efficacy of a Brucella melitensis vaccine in mice, even without increasing specific antibody levels []. This suggests a broader immunostimulatory effect beyond simply boosting antibody production.
Q19: Is there evidence of anthelmintic resistance to this compound?
A19: Yes, unfortunately, studies have reported emerging resistance to this compound in certain parasite populations. A study in Haryana, India, found resistance in Haemonchus contortus, a prevalent sheep nematode, to this compound, highlighting the growing concern of anthelmintic resistance [].
Q20: How does this compound resistance relate to other anthelmintics?
A20: Research shows that parasite resistance to one anthelmintic class can often be associated with resistance to others. Studies have observed simultaneous resistance to this compound, albendazole, and ivermectin in gastrointestinal nematodes of goats, indicating a potential for cross-resistance among these commonly used drugs [, ]. This emphasizes the need for sustainable parasite control strategies to mitigate the development and spread of resistance.
Q21: Are there any reported toxic effects of this compound?
A21: While generally considered safe at therapeutic doses, some studies have documented mild and transient side effects in animals, including nervous and locomotor excitement, tremors, salivation, and increased respiration and pulse rate []. The research emphasizes the importance of using appropriate dosages to minimize the risk of adverse effects.
Q22: Have there been attempts to improve this compound delivery?
A22: Yes, one study demonstrated the benefits of using a zeolite matrix to enable the slow release of this compound, leading to enhanced efficacy against Nippostrongylus brasiliensis in rats []. This highlights the potential of drug delivery strategies to improve this compound's therapeutic profile.
Q23: How is this compound quantified in various matrices?
A23: Several analytical methods are employed to quantify this compound, including:
- High-Performance Liquid Chromatography (HPLC): HPLC, coupled with UV detection, is commonly used for separating and quantifying this compound in pharmaceutical formulations and biological samples [, , , ].
- Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS): This highly sensitive and specific technique enables accurate quantification of this compound and its enantiomers (levamisole and dexamisole) in complex matrices like hair and serum samples [, ].
- Polarimetry: This traditional method, while less sensitive than chromatographic techniques, can be used to determine the enantiomeric purity of levamisole by measuring its specific rotation [].
Q24: Does the research discuss the environmental fate of this compound?
A24: The provided research primarily focuses on the pharmaceutical and pharmacological aspects of this compound and does not provide detailed information on its environmental impact or degradation pathways. Further research is needed to assess its potential ecotoxicological effects and explore strategies for mitigating any negative environmental impacts.
Q25: Are there studies on the dissolution and solubility of this compound?
A25: While the provided research does not specifically focus on this compound's dissolution and solubility, one study utilized a spectrofluorimetric method with a biological dye (Felumin) to track the drug's behavior in different solutions []. This approach highlights the importance of understanding this compound's solubility and complex formation in various media for analytical and formulation purposes.
Q26: How are analytical methods for this compound validated?
A26: Researchers adhere to established guidelines, such as those outlined by the International Conference on Harmonization (ICH), to validate analytical methods for this compound quantification. These validation procedures typically involve assessing parameters like:
Q27: What quality control measures are important for this compound?
A27: Ensuring the quality, safety, and efficacy of this compound throughout its lifecycle involves rigorous quality control measures during development, manufacturing, and distribution. These measures may include:
Q28: Does this compound induce any immunological responses?
A28: Research indicates that this compound exhibits immunomodulatory properties, influencing immune responses in various ways. For instance, it enhanced the effectiveness of a Brucella melitensis vaccine in mice [], suggesting its potential as an immunostimulant.
Q29: Is there information on drug-transporter interactions, drug-metabolizing enzyme induction/inhibition, biocompatibility, or biodegradability of this compound?
A29: The provided research does not delve into the specifics of this compound's interactions with drug transporters or metabolizing enzymes, nor does it discuss its biocompatibility or biodegradability. Further investigation is needed to understand these aspects fully.
Q30: Does the research discuss alternatives to this compound, recycling strategies, or specific research infrastructure for its study?
A30: The research provided focuses primarily on this compound's properties and efficacy. It does not provide comparative analyses of alternative anthelmintics, discuss recycling strategies for the drug or its waste products, or highlight specific research infrastructure dedicated to its study. Further exploration of these areas is necessary.
Q31: What are the historical milestones in this compound research?
A31: Key historical milestones in this compound research, based on the provided papers, include:
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。

![Bicyclo[3.1.0]hexane](/img/structure/B1196588.png)