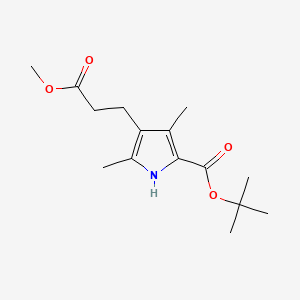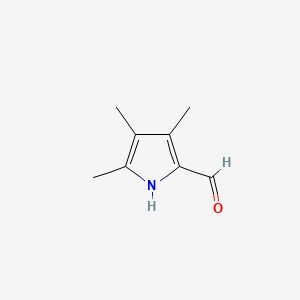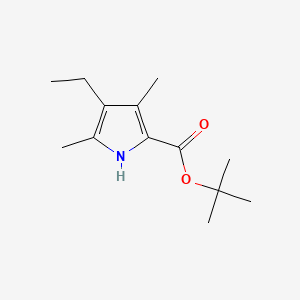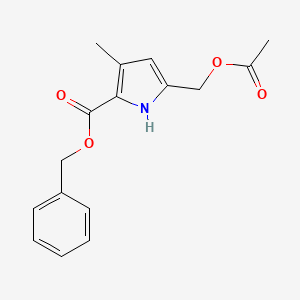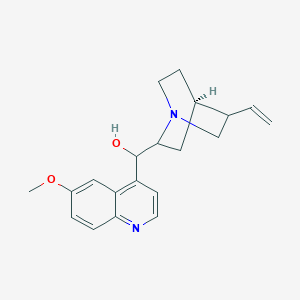
キニーネ
概要
説明
キニーネは、南米のアンデス地方原産のキナノキの樹皮から得られる天然のアルカロイドです。歴史的には、マラリア治療薬として使用されてきました。また、苦味で知られており、トニックウォーターにも使用されています。 キニーネは1820年に初めて単離され、特に合成マラリア治療薬が登場する前はマラリア治療に重要な役割を果たしてきました .
2. 製法
合成経路と反応条件: キニーネの全合成は、有機化学における重要なマイルストーンでした。最初の立体選択的合成は、2001年にギルバート・ストークによって達成されました。 合成経路は、キノトキシン形成を含む複数のステップからなり、その後一連の反応を経てキニーネに変換されます .
工業生産方法: キニーネの工業生産は、主にキナノキの樹皮からの抽出によって行われます。樹皮を採取し、乾燥させた後、一連の抽出と精製プロセスを経てキニーネを単離します。 抽出されたキニーネは、その後、硫酸キニーネや塩酸キニーネなどの様々な塩に変換され、医薬品として使用されます .
作用機序
キニーネは、寄生虫がヘモグロビンを消化する能力を阻害することによって、マラリア治療効果を発揮します。キニーネは、ヘモグロビン消化の有毒な副産物であるヘモゾインの形成を阻害し、寄生虫にとって有毒な遊離ヘムの蓄積につながります。 これにより、マラリア原虫が死滅します . さらに、キニーネは筋肉膜とナトリウムチャネルに影響を与え、これが筋肉疾患の治療におけるキニーネの使用を説明しています .
類似化合物:
クロロキン: マラリア原虫の血液型を抑制する効果がより高い別のマラリア治療薬。
アルテメターとルメラントリン: これらは、マラリアの併用療法で使用されており、キニーネとは異なる作用機序を持っています.
キニーネの独自性: キニーネの独特の作用機序、抵抗性の発達速度が遅いこと、感染症の治療に使用された最初の化学化合物の1つとしての歴史的重要性により、キニーネは重要な化合物となっています。 合成マラリア治療薬とは異なり、キニーネは天然資源から得られており、より幅広い用途があります .
科学的研究の応用
Quinine has a wide range of applications in scientific research:
Chemistry: It serves as a chiral catalyst in asymmetric synthesis.
Biology: Quinine is used to study the effects of alkaloids on biological systems.
生化学分析
Biochemical Properties
Quinine plays a role in biochemical reactions as an electron carrier . It has the potential to bind to thiol, amine, and hydroxyl groups . These properties make quinine a biologically active compound .
Cellular Effects
Quinine is used to treat life-threatening infections caused by chloroquine-resistant Plasmodium falciparum malaria . It acts as a blood schizonticide and also has gametocytocidal activity against P. vivax and P. malariae . As a weak base, it is concentrated in the food vacuoles of P. falciparum .
Molecular Mechanism
It is known that quinine interferes with the parasite’s ability to break down and digest hemoglobin . Consequently, the parasite is poisoned by its own waste product (hemozoin) .
Temporal Effects in Laboratory Settings
It is known that quinine is a stable compound and can be used for quantitative analysis in liquids .
Dosage Effects in Animal Models
It is known that quinine has a narrow therapeutic index, with a large number of side effects at higher doses .
Metabolic Pathways
Quinine is metabolized in the liver by the cytochrome P450 system into several metabolites that are excreted in the urine
Transport and Distribution
Quinine is rapidly absorbed from the gastrointestinal tract and distributed throughout the body tissues . It is also known to cross the placenta and can be found in the fetus .
Subcellular Localization
Due to its weak base properties, it is known to accumulate in acidic compartments of the cell, such as food vacuoles of the malaria parasite .
準備方法
Synthetic Routes and Reaction Conditions: The total synthesis of quinine has been a significant milestone in organic chemistry. The first stereoselective total synthesis was achieved by Gilbert Stork in 2001. The synthetic route involves multiple steps, including the formation of quinotoxine, which is then converted to quinine through a series of reactions .
Industrial Production Methods: Industrial production of quinine primarily involves extraction from the bark of the cinchona tree. The bark is harvested, dried, and then subjected to a series of extraction and purification processes to isolate quinine. The extracted quinine is then converted into various salts, such as quinine sulfate or quinine hydrochloride, for medicinal use .
化学反応の分析
反応の種類: キニーネは、酸化、還元、置換など、様々な化学反応を起こします。 例えば、酸化してキノトキシンを形成することができ、キノトキシンはさらに還元されてキニーネを再生することができます .
一般的な試薬と条件:
酸化: キニーネをキノトキシンに酸化する際には、次亜臭素酸ナトリウムなどの試薬が使用されます。
還元: エタノール中のアルミニウム粉末とエトキシドナトリウムは、キノトキシンをキニーネに還元するために一般的に使用されます.
主な生成物: これらの反応で生成される主な生成物には、キノトキシンやその他の誘導体が含まれ、その生成物は使用される特定の反応条件や試薬によって異なります .
4. 科学研究への応用
キニーネは、科学研究において幅広い用途があります。
化学: キニーネは、不斉合成においてキラル触媒として役立ちます。
生物学: キニーネは、アルカロイドが生物系に与える影響を研究するために使用されます。
医学: マラリア治療効果に加えて、キニーネはバベシア症の治療にも使用されており、夜間脚のけいれんや先天性ミオトニアの治療における可能性についても調査されています.
類似化合物との比較
Chloroquine: Another antimalarial drug that is more effective in suppressing the growth of blood forms of the malaria parasite.
Artemether and Lumefantrine: These are used in combination therapies for malaria and have different mechanisms of action compared to quinine.
Uniqueness of Quinine: Quinine’s unique mechanism of action, slow development of resistance, and its historical significance as one of the first chemical compounds used to treat an infectious disease highlight its importance. Unlike synthetic antimalarials, quinine is derived from a natural source and has a broader range of applications .
特性
IUPAC Name |
(R)-[(2S,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl]-(6-methoxyquinolin-4-yl)methanol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19-,20+/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
LOUPRKONTZGTKE-WZBLMQSHSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
COC1=CC2=C(C=CN=C2C=C1)C(C3CC4CCN3CC4C=C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
COC1=CC2=C(C=CN=C2C=C1)[C@H]([C@@H]3C[C@@H]4CCN3C[C@@H]4C=C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C20H24N2O2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID0044280 | |
| Record name | Quinine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0044280 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
324.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Quinine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014611 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
In water, 500 mg/L at 15 °C, 1 g dissolves in: 1900 mL water, 760 mL boiling water, 1 g dissolves in: 80 mL benzene (18 mL at 50 °C), 1.2 mL chloroform, 250 mL dry ether, 20 mL glycerol, 0.8 mL alcohol, 1900 mL of 10% ammonia water; almost insoluble in petroleum ether, Soluble in ether, chloroform, carbon disulfide, glycerol, alkalies, and acids (with formation of salts), Sol in pyrimidine, 3.34e-01 g/L | |
| Record name | Quinine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00468 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | QUININE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/2501 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Quinine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014611 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
The theorized mechanism of action for quinine and related anti-malarial drugs is that these drugs are toxic to the malaria parasite. Specifically, the drugs interfere with the parasite's ability to break down and digest hemoglobin. Consequently, the parasite starves and/or builds up toxic levels of partially degraded hemoglobin in itself., Quinine has a local anesthetic action and analgesic, antipyretic, and oxytocic effects. Quinine also has cardiovascular effects similar to those of quinidine. | |
| Record name | Quinine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00468 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | QUININE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/2501 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Triboluminescent, orthorhombic needles from absolute alcohol, Bulky, white, amorphous powder or crystalline alkaloid, CRYSTALS TURN BROWN ON EXPOSURE TO AIR | |
CAS No. |
72402-53-0, 130-95-0, 1407-83-6 | |
| Record name | (8α,9R)-(±)-6′-Methoxycinchonan-9-ol | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=72402-53-0 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Quinine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=130-95-0 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Quinine [BAN:NF] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000130950 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Quinine tannate [USP] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0001407836 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Quinine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00468 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Cinchonan-9-ol, 6'-methoxy-, (8.alpha.,9R)- | |
| Source | EPA Chemicals under the TSCA | |
| URL | https://www.epa.gov/chemicals-under-tsca | |
| Description | EPA Chemicals under the Toxic Substances Control Act (TSCA) collection contains information on chemicals and their regulations under TSCA, including non-confidential content from the TSCA Chemical Substance Inventory and Chemical Data Reporting. | |
| Record name | Quinine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0044280 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Quinine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.004.550 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | QUININE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/A7V27PHC7A | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | QUININE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/2501 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Quinine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014611 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
177 °C (some decomposition), Microcrystalline powder; mp: 57 °C; efflorescent; loses one water molecule in air, two water molecules over sulfuric acid; anhydrous at 125 °C /Quinine trihydrate/, 57 °C | |
| Record name | Quinine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00468 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | QUININE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/2501 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Quinine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014611 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary mechanism of action of quinine against malaria?
A1: While the precise mechanism remains incompletely understood, quinine is known to exert its antimalarial effects by interfering with the parasite's ability to metabolize and detoxify heme. [] During its lifecycle within red blood cells, Plasmodium falciparum degrades hemoglobin, releasing toxic heme. Quinine accumulates in the parasite's digestive vacuole, forming complexes with heme and preventing its detoxification into hemozoin. This leads to a buildup of toxic heme, ultimately killing the parasite. []
Q2: How does quinine affect muscle cramps?
A2: The mechanism by which quinine relieves muscle cramps is not fully elucidated. Research suggests that it might interfere with the excitability of muscle fibers, reducing their tendency to contract involuntarily and cause cramps. [] Studies have shown that quinine can block acetylcholine-evoked responses in human muscle nicotinic acetylcholine receptors (nAChRs), suggesting a potential mechanism for its anti-cramping effects. []
Q3: Does quinine affect tryptophan levels in cells?
A3: Yes, research indicates that quinine can disrupt tryptophan transport within cells. It inhibits the uptake of tryptophan by associating with the high-affinity tryptophan/tyrosine permease, Tat2p. This association is suppressible by tryptophan, indicating a competitive interaction. The inhibition of tryptophan uptake by quinine can lead to tryptophan starvation in cells. []
Q4: What is the molecular formula and weight of quinine?
A4: The molecular formula of quinine is C20H24N2O2, and its molecular weight is 324.42 g/mol. []
Q5: Are there any spectroscopic techniques used to characterize quinine?
A5: Yes, various spectroscopic techniques, including Fourier transform infrared (FTIR) spectroscopy, nuclear magnetic resonance (NMR) spectroscopy, and mass spectrometry (MS), have been employed to characterize quinine and its derivatives. FTIR helps identify functional groups, while NMR provides insights into the molecule's structure and connectivity. MS confirms the molecular weight and fragmentation patterns, aiding in structural elucidation. [, , ]
Q6: Does quinine possess any catalytic properties?
A6: While not traditionally considered a catalyst, quinine and its derivatives have found applications in asymmetric synthesis as chiral ligands or organocatalysts. Their unique structural features, characterized by multiple chiral centers and a tertiary amine, enable them to influence the stereochemical outcome of reactions. [, ]
Q7: Have there been any computational studies conducted on quinine?
A7: Yes, computational chemistry approaches have been employed to study quinine's interactions with biological targets and to develop quantitative structure-activity relationship (QSAR) models. Molecular docking studies have provided insights into the binding modes of quinine with enzymes and receptors, while QSAR models have helped predict the antimalarial activity of quinine analogs based on their structural features. []
Q8: How do modifications to the quinine structure impact its activity?
A8: SAR studies have revealed that specific structural features are crucial for quinine's activity. The stereochemistry at the C-9 position significantly influences antimalarial potency, with quinine and quinidine exhibiting higher activity than their 9-epi counterparts. Modifications to the quinoline and quinuclidine rings, as well as the hydroxyl group, have also been explored to understand their impact on antimalarial activity, toxicity, and physicochemical properties. [, ]
Q9: How is quinine metabolized in the body?
A9: Quinine is primarily metabolized in the liver via oxidative pathways, involving cytochrome P450 enzymes. Major metabolites include 3-hydroxyquinine and 10,11-dihydroxydihydroquinine. The metabolic profile of quinine can be influenced by factors like genetic polymorphisms in drug-metabolizing enzymes, co-administration of other drugs, and liver function. [, ]
Q10: How does renal function affect quinine elimination?
A10: Renal impairment can significantly impact the elimination of quinine, as it is primarily excreted in the urine. In patients with chronic renal failure, particularly those on hemodialysis, the clearance of free (unbound) quinine is increased, leading to lower plasma concentrations. This altered pharmacokinetic profile necessitates careful dose adjustments to avoid toxicity. []
Q11: What types of in vitro and in vivo models are used to study quinine's activity?
A11: In vitro studies often utilize cultures of Plasmodium falciparum to determine the drug's efficacy in inhibiting parasite growth. Animal models, such as mice infected with malaria, are used to evaluate in vivo efficacy, pharmacokinetic properties, and potential toxicity. Clinical trials in humans are essential for assessing the drug's safety and efficacy in treating malaria. [, ]
Q12: What are the mechanisms of resistance to quinine in malaria parasites?
A12: Resistance to quinine in Plasmodium falciparum is a complex phenomenon, often involving mutations in genes encoding proteins involved in drug transport or drug targets. Mutations in the Pfmdr1 gene, encoding a transmembrane protein involved in drug efflux, have been associated with decreased susceptibility to quinine. []
Q13: What are the potential adverse effects of quinine?
A13: While generally well-tolerated at therapeutic doses, quinine can cause a range of adverse effects, including gastrointestinal disturbances, tinnitus, headache, and visual disturbances. In rare cases, it can lead to serious complications such as hemolytic anemia, thrombocytopenia, and hypoglycemia. Careful monitoring for adverse events is essential, especially in patients with underlying medical conditions. [, , , , ]
Q14: Are there strategies to improve the delivery of quinine to specific targets?
A14: Researchers are exploring various drug delivery approaches to enhance the targeting and efficacy of antimalarial drugs like quinine. Nanoparticle-based delivery systems, for example, hold promise in improving drug solubility, bioavailability, and targeted delivery to infected red blood cells, potentially reducing toxicity and improving therapeutic outcomes. []
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。


![10-Hydroxy-4,4,6a,12b-tetramethyl-1,2,3,4,4a,5,6,6a,12a,12b-decahydro-9h-benzo[a]xanthen-9-one](/img/structure/B1679877.png)