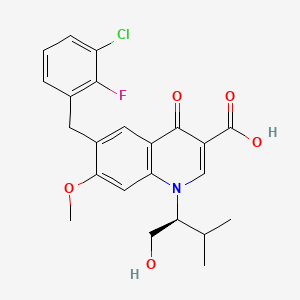
エルビテグラビル
説明
Elvitegravir is a quinolinemonocarboxylic acid that is 7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid substited at position 1 by a 1-hydroxy-3-methylbutan-2-yl group and at position 6 by a 3-chloro-2-fluorobenzyl group (the S-enantiomer). It is used in combination therapy for the treatment of HIV-1 infection. It has a role as a HIV-1 integrase inhibitor. It is a quinolinemonocarboxylic acid, an organofluorine compound, an aromatic ether, a quinolone and a member of monochlorobenzenes.
Elvitegravir is a human immunodeficiency virus type 1 (HIV-1) integrase strand transfer inhibitor (INSTI) used for the treatment of HIV-1 infection in antiretroviral treatment-experienced adults. Because integrase is necessary for viral replication, inhibition prevents the integration of HIV-1 DNA into the host genome and thereby blocks the formation of the HIV-1 provirus and resulting propagation of the viral infection. Although available as a single dose tablet, elvitegravir must be used in combination with an HIV protease inhibitor coadministered with ritonavir and another antiretroviral drug. Elvitegravir was first licensed from Japan Tobacco in 2008 and developed by Gilead Sciences. It was FDA approved on August 27, 2012. On September 24, 2014, the FDA approved the single pill form of elvitegravir.
Elvitegravir is a Human Immunodeficiency Virus Integrase Strand Transfer Inhibitor. The mechanism of action of elvitegravir is as a HIV Integrase Inhibitor, and Cytochrome P450 2C9 Inducer.
Elvitegravir is a modified quinolone antibiotic with activity against human immunodeficiency virus 1. Elvitegravir is an inhibitor of viral integrase and retains activity against integrase mutants that are resistant to Raltegravir.
See also: ... View More ...
科学的研究の応用
エルビテグラビル:科学研究アプリケーションの包括的な分析
HIV-1 感染治療: エルビテグラビルは、主に他の抗レトロウイルス薬と組み合わせて、以前に治療を受けた成人の HIV-1 感染の治療に使用されます。 これは、HIV インテグラーゼ阻害剤として機能し、ウイルスがその遺伝物質を宿主細胞の DNA に組み込む能力を阻害します。これは、ウイルス複製過程における重要なステップです .
HIV 株に対する抗ウイルス活性: 研究によると、エルビテグラビルは、ラボの HIV-1 株だけでなく、HIV-2 株に対しても強力な抗ウイルス活性を示すことが示されています。 この広域スペクトル活性により、さまざまな形式の HIV ウイルスに対する闘いにおいて貴重な要素となっています .
薬剤開発と特性評価: エルビテグラビルは、その特性と関連する不純物を特徴付けるために広範な研究の対象となっています。これは、薬剤開発における品質管理にとって不可欠です。 NMR や LC-MS 分光法などの技術を使用して、その構造を研究し、プロセス関連の不純物を特定しています .
薬物動態とバイオアッセイ開発: エルビテグラビルの薬物動態を理解するための研究が行われています。これは、その投与量と投与頻度を決定するために不可欠です。 さらに、分子インプリントポリマーを使用したバイオアッセイが、エルビテグラビルの特異的な抽出と測定のために開発されており、その分析と品質保証に役立っています .
臨床試験と比較研究: エルビテグラビルは、HIV-1 感染者の最適化されたバックグラウンド療法と組み合わせた場合、他のプロテアーゼ阻害剤と比較してその有効性を評価するために、臨床試験で評価されてきました。 これらの研究は、治療レジメンにおけるその位置を確立し、治療の決定を導くのに役立ちます .
製剤研究: 崩壊時間、残留物、漏れ、および許容性に関するさまざまなインサートプロトタイプなど、エルビテグラビルのさまざまな製剤に関する研究が進行中です。 この研究の目的は、送達方法を最適化し、患者のコンプライアンスを向上させることです .
作用機序
Target of Action
Elvitegravir is an antiretroviral agent that primarily targets the HIV-1 integrase . This enzyme is encoded by the HIV-1 virus and is essential for viral replication .
Mode of Action
Elvitegravir acts as an integrase strand transfer inhibitor (INSTI) . The integrase enzyme is responsible for integrating the HIV-1 DNA into the host genome. By inhibiting this enzyme, elvitegravir prevents the integration of HIV-1 DNA into the host genome, thereby blocking the formation of the HIV-1 provirus and the propagation of the viral infection .
Biochemical Pathways
The primary biochemical pathway affected by elvitegravir is the HIV-1 replication cycle . By inhibiting the integrase enzyme, elvitegravir disrupts the integration of the viral DNA into the host genome, a crucial step in the HIV-1 replication cycle . This results in the prevention of the formation of new HIV-1 proviruses, thereby halting the propagation of the viral infection .
Pharmacokinetics
Elvitegravir undergoes primarily oxidative metabolism via CYP3A , and is secondarily glucuronidated via UGT1A1/3 enzymes . The metabolites of elvitegravir are found in the plasma at very low concentrations and display considerably lower anti-HIV activity . Elvitegravir’s metabolism primarily occurs via cytochrome P450 3A4 (CYP3A4) and requires pharmacokinetic boosting to achieve systemic exposures amenable to once-daily dosing .
Result of Action
The molecular effect of elvitegravir’s action is the inhibition of the HIV-1 integrase enzyme, which prevents the integration of HIV-1 DNA into the host genome . On a cellular level, this results in the blocking of the formation of the HIV-1 provirus and the propagation of the viral infection . This effectively halts the replication of the HIV-1 virus within the host cells .
Action Environment
Environmental factors such as the presence of other drugs can influence the action of elvitegravir. For instance, elvitegravir must be used in combination with an HIV protease inhibitor coadministered with ritonavir and another antiretroviral drug . Additionally, substances that induce CYP3A can reduce elvitegravir concentrations in the body, potentially triggering the development of resistant virus strains . Furthermore, decreased levels of plasma albumin, which occur during pregnancy, can enhance the hepatic clearance of elvitegravir, as it binds strongly to plasma albumin .
特性
IUPAC Name |
6-[(3-chloro-2-fluorophenyl)methyl]-1-[(2S)-1-hydroxy-3-methylbutan-2-yl]-7-methoxy-4-oxoquinoline-3-carboxylic acid | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C23H23ClFNO5/c1-12(2)19(11-27)26-10-16(23(29)30)22(28)15-8-14(20(31-3)9-18(15)26)7-13-5-4-6-17(24)21(13)25/h4-6,8-10,12,19,27H,7,11H2,1-3H3,(H,29,30)/t19-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
JUZYLCPPVHEVSV-LJQANCHMSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)C(CO)N1C=C(C(=O)C2=C1C=C(C(=C2)CC3=C(C(=CC=C3)Cl)F)OC)C(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC(C)[C@@H](CO)N1C=C(C(=O)C2=C1C=C(C(=C2)CC3=C(C(=CC=C3)Cl)F)OC)C(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C23H23ClFNO5 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID101021650 | |
| Record name | Elvitegravir | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID101021650 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
447.9 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
<0.3 mcg/mL | |
| Record name | Elvitegravir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09101 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Mechanism of Action |
Elvitegravir is an HIV-1 integrase strand transfer inhibitor (INSTI). Integrase is an HIV-1 encoded enzyme that is required for viral replication. Inhibition of integrase prevents the integration of HIV-1 DNA into host genomic DNA, blocking the formation of the HIV-1 provirus and propagation of the viral infection. Elvitegravir does not inhibit human topoisomerases I or II. | |
| Record name | Elvitegravir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09101 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
697761-98-1 | |
| Record name | Elvitegravir | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=697761-98-1 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Elvitegravir [USAN:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0697761981 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Elvitegravir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09101 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Elvitegravir | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID101021650 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 6-(3-chloro-2-fluorobenzyl)-1-[(2S)-1-hydroxy-3-methylbutan-2-yl]-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | ELVITEGRAVIR | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/4GDQ854U53 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。