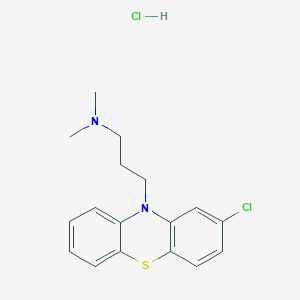
氯丙嗪盐酸盐
描述
氯丙嗪盐酸盐是一种强效的合成镇静药物,它作为中枢神经系统的抑制剂,选择性地作用于大脑的高级中枢。 它主要用于治疗精神疾病,如精神分裂症、双相情感障碍、儿童严重的行为问题、恶心和呕吐、手术前的焦虑和持续性呃逆 .
科学研究应用
氯丙嗪盐酸盐在科学研究中具有广泛的应用:
作用机制
氯丙嗪盐酸盐主要作为多巴胺受体拮抗剂。它结合并阻断大脑中的多巴胺受体,特别是 D2 受体,降低多巴胺信号通路的活性。这种作用被认为是其抗精神病作用的原因。 此外,氯丙嗪盐酸盐还表现出抗血清素和抗组胺活性,这有助于其整体治疗特性 .
生化分析
Biochemical Properties
Chlorpromazine hydrochloride plays a crucial role in biochemical reactions by acting as an antagonist on various postsynaptic receptors. It primarily interacts with dopaminergic receptors (subtypes D1, D2, D3, and D4), serotonergic receptors (5-HT1 and 5-HT2), histaminergic receptors (H1 and H2), and muscarinic receptors (M1 and M2) . These interactions result in the modulation of neurotransmitter activity, leading to its antipsychotic, antiemetic, and sedative effects.
Cellular Effects
Chlorpromazine hydrochloride exerts significant effects on various types of cells and cellular processes. It influences cell function by modulating cell signaling pathways, gene expression, and cellular metabolism. For instance, chlorpromazine hydrochloride can initiate cell death in both cancerous and healthy liver cells by inducing apoptosis . Additionally, it affects neurogenesis in the rat brain by decreasing the number of Sox-2-expressing stem cells and early neural progenitors .
Molecular Mechanism
The molecular mechanism of chlorpromazine hydrochloride involves its action as an antagonist on different postsynaptic receptors. It blocks dopaminergic receptors, leading to a reduction in dopamine activity, which is associated with its antipsychotic properties . Chlorpromazine hydrochloride also inhibits serotonergic, histaminergic, and muscarinic receptors, contributing to its anxiolytic, antidepressive, and antiaggressive effects . These interactions result in changes in gene expression and enzyme activity, further influencing cellular functions.
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of chlorpromazine hydrochloride change over time. The compound exhibits multicompartmental pharmacokinetics, with a half-life of approximately 11.05 hours . It undergoes extensive first-pass metabolism, leading to significant variability in its pharmacokinetics. Over time, chlorpromazine hydrochloride can degrade, affecting its stability and long-term impact on cellular functions .
Dosage Effects in Animal Models
The effects of chlorpromazine hydrochloride vary with different dosages in animal models. At therapeutic doses, it can cause side effects such as orthostatic hypotension, jaundice, and leukopenia . High doses may lead to toxic effects, including interference with pituitary-gonadal function and neurogenic stem/progenitor cell formation . These dosage-dependent effects highlight the importance of careful dosage management in clinical settings.
Metabolic Pathways
Chlorpromazine hydrochloride is involved in several metabolic pathways, primarily catalyzed by cytochrome P450 isoenzymes. The main metabolic mechanisms include N-demethylation, S-oxidation, and aromatic hydroxylation . These pathways result in the formation of various metabolites, such as mono-N-desmethylchlorpromazine, chlorpromazine sulfoxide, and 7-hydroxychlorpromazine . These metabolites can further undergo phase II metabolism, including glucuronidation and sulfation .
Transport and Distribution
Chlorpromazine hydrochloride is transported and distributed within cells and tissues through various mechanisms. It exhibits wide distribution in the body, with a volume of distribution at steady state of approximately 642 liters . The compound is highly protein-bound, with 90-99% of it bound to plasma proteins . This extensive binding affects its localization and accumulation in different tissues.
Subcellular Localization
The subcellular localization of chlorpromazine hydrochloride is influenced by its interactions with various biomolecules. It can localize to different cellular compartments, including the cytoplasm and cell membrane. The compound’s activity and function are affected by its localization, as it can interact with specific receptors and enzymes within these compartments . Post-translational modifications, such as phosphorylation, may also play a role in directing chlorpromazine hydrochloride to specific organelles.
准备方法
合成路线和反应条件
氯丙嗪盐酸盐通过多步合成过程制备。合成从 2-氯吩噻嗪与 3-二甲氨基丙基氯在碱(如氨基钠)存在下的反应开始。 该反应生成氯丙嗪,然后用盐酸处理将其转化为盐酸盐 .
工业生产方法
在工业环境中,氯丙嗪盐酸盐采用类似的合成路线,但规模更大。该过程包括在特定条件下将氯丙嗪与微晶纤维素、乳糖一水合物、预糊化淀粉、胶体二氧化硅和硬脂酸镁混合。 然后对混合物进行筛分和压制,得到最终的药物制剂 .
化学反应分析
反应类型
氯丙嗪盐酸盐会发生多种化学反应,包括氧化、还原和取代反应。
常用试剂和条件
氧化: 氯丙嗪盐酸盐可以在酸性条件下用高锰酸钾或过氧化氢等试剂氧化。
还原: 还原可以用氢化锂铝或硼氢化钠等试剂实现。
取代: 取代反应通常涉及亲核试剂,如氢氧根离子或胺类,在碱性条件下进行。
主要产物
相似化合物的比较
氯丙嗪盐酸盐属于吩噻嗪类抗精神病药物。类似的化合物包括:
丙嗪: 另一种吩噻嗪类抗精神病药物,具有类似的作用机制,但药效不如氯丙嗪.
硫利达嗪: 一种吩噻嗪衍生物,具有类似的抗精神病特性,但副作用特征不同。
氟奋乃静: 一种更强的吩噻嗪类抗精神病药物,用于治疗精神分裂症。
属性
IUPAC Name |
3-(2-chlorophenothiazin-10-yl)-N,N-dimethylpropan-1-amine;hydrochloride | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C17H19ClN2S.ClH/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20;/h3-4,6-9,12H,5,10-11H2,1-2H3;1H | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
FBSMERQALIEGJT-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CN(C)CCCN1C2=CC=CC=C2SC3=C1C=C(C=C3)Cl.Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C17H19ClN2S.ClH, C17H20Cl2N2S | |
| Record name | CHLORPROMAZINE HYDROCHLORIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20027 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID7024827 | |
| Record name | Chlorpromazine hydrochloride | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7024827 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
355.3 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Chlorpromazine hydrochloride appears as white or creamy-white odorless crystalline powder with very bitter taste. pH (5% aqueous solution) 4.0-5.5. pH (10% aqueous solution) 4-5. (NTP, 1992) | |
| Record name | CHLORPROMAZINE HYDROCHLORIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20027 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
Solubility |
greater than or equal to 100 mg/mL at 75 °F (NTP, 1992) | |
| Record name | CHLORPROMAZINE HYDROCHLORIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20027 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
CAS No. |
69-09-0 | |
| Record name | CHLORPROMAZINE HYDROCHLORIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20027 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Chlorpromazine hydrochloride | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=69-09-0 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Chlorpromazine hydrochloride [USP:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000069090 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Chlorpromazine hydrochloride | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=17479 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | 10H-Phenothiazine-10-propanamine, 2-chloro-N,N-dimethyl-, hydrochloride (1:1) | |
| Source | EPA Chemicals under the TSCA | |
| URL | https://www.epa.gov/chemicals-under-tsca | |
| Description | EPA Chemicals under the Toxic Substances Control Act (TSCA) collection contains information on chemicals and their regulations under TSCA, including non-confidential content from the TSCA Chemical Substance Inventory and Chemical Data Reporting. | |
| Record name | Chlorpromazine hydrochloride | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7024827 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Chlorpromazine hydrochloride | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.000.638 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | CHLORPROMAZINE HYDROCHLORIDE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/9WP59609J6 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Melting Point |
378 to 385 °F (decomposes) (NTP, 1992) | |
| Record name | CHLORPROMAZINE HYDROCHLORIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20027 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary mechanism of action of Chlorpromazine Hydrochloride?
A1: Chlorpromazine Hydrochloride primarily acts as a dopamine antagonist, blocking the action of dopamine in the brain. This is thought to be responsible for its antipsychotic effects. []
Q2: How does Chlorpromazine Hydrochloride affect prolactin levels?
A2: Chlorpromazine Hydrochloride can stimulate prolactin release in rats, with peak levels observed between 30 and 90 minutes after administration. This effect is dose-dependent and correlates with the drug's antipsychotic potency in humans. []
Q3: Does Chlorpromazine Hydrochloride impact bile secretion?
A3: Yes, Chlorpromazine Hydrochloride can induce cholestasis, a condition characterized by reduced bile flow. This effect is likely due to the drug's ability to form insoluble complexes with bile salts, leading to their precipitation and reduced secretion. [] Studies in Rhesus monkeys demonstrate that this effect is dose-dependent and reversible, with bile flow returning to normal as drug concentrations decrease. []
Q4: What is the molecular formula and weight of Chlorpromazine Hydrochloride?
A4: The molecular formula of Chlorpromazine Hydrochloride is C17H19ClN2S·HCl, and its molecular weight is 355.33 g/mol.
Q5: Does Chlorpromazine Hydrochloride interact with lipids?
A5: Yes, being an amphiphilic molecule, Chlorpromazine Hydrochloride can interact with both polar and non-polar molecules. Studies show that it can interact with bile lipids, forming insoluble complexes with bile salts and solubilizing membrane phospholipids like phosphatidylserine and phosphatidylcholine. []
Q6: How does the structure of Chlorpromazine Hydrochloride contribute to its interaction with bile salts?
A6: Chlorpromazine Hydrochloride is amphiphilic due to its polar tertiary amine group linked to a hydrophobic tricyclic ring system by a short paraffin chain. This structure allows it to interact with both the hydrophilic and hydrophobic regions of bile salts, leading to complex formation and precipitation. []
Q7: Does the sugar-coating on Chlorpromazine Hydrochloride tablets interfere with content determination?
A7: No, studies have shown that the sugar-coating does not interfere with UV spectrophotometric determination of Chlorpromazine Hydrochloride content in tablets. Therefore, shelling the sugar-coat is unnecessary for accurate analysis. []
Q8: What is the impact of Chlorpromazine Hydrochloride on regional blood flow in acute cerebral infarction patients?
A8: Studies show that Chlorpromazine Hydrochloride, particularly when combined with ozagrel, can significantly improve regional blood flow speed in acute cerebral infarction patients. This combination also effectively reduces blood viscosity. []
Q9: What effect does Chlorpromazine Hydrochloride have on diffuse large B lymphoma cells?
A9: In vitro studies show that Chlorpromazine Hydrochloride can inhibit the proliferation of diffuse large B lymphoma cells, potentially by promoting the expression of S1PR2. It also induces apoptosis and promotes G1 cell cycle arrest in these cells. []
Q10: Has Chlorpromazine Hydrochloride shown efficacy in treating intractable hiccups?
A10: Yes, clinical observations suggest that Chlorpromazine Hydrochloride is effective in treating intractable hiccups, showing positive results through various administration routes like acupoint injection, nasal mucosa medication, and combined with traditional Chinese medicine. [, , ]
Q11: What analytical methods are commonly employed for Chlorpromazine Hydrochloride quantification?
A11: Several analytical methods are used for Chlorpromazine Hydrochloride quantification, including:
- Spectrophotometry: This method relies on the drug's absorbance at specific wavelengths, but it can be influenced by excipients or degradation products. [, , , , , , , , ]
- Spectrofluorimetry: This method measures the drug's fluorescence intensity, offering high sensitivity and a low detection limit. [, ]
- Chemiluminescence: This technique exploits the light emitted during a chemical reaction involving the drug, offering high sensitivity. [, ]
- High-performance liquid chromatography (HPLC): This method separates the drug from other components in a sample before detection, offering high selectivity and sensitivity. [, ]
- Ultra performance liquid chromatography-tandem mass spectrometry (UPLC/MS/MS): This method combines the separation power of HPLC with the sensitivity and selectivity of mass spectrometry. []
- Flow injection analysis: This technique automates sample injection and analysis, improving speed and precision. [, , , ]
- Resonance scattering spectrometry: This method measures the scattering of light by nanoparticles formed upon reaction with the drug, offering high sensitivity and selectivity. []
Q12: Are there specific challenges in analyzing Chlorpromazine Hydrochloride in biological samples?
A12: Yes, biological matrices can introduce interferences, requiring extraction and cleanup procedures before analysis. Methods like UPLC/MS/MS are particularly useful for analyzing trace levels in complex matrices like animal tissues. []
Q13: How do analytical methods ensure accurate and reliable determination of Chlorpromazine Hydrochloride?
A13: Analytical methods undergo validation to ensure their accuracy, precision, and specificity for Chlorpromazine Hydrochloride determination. This involves assessing parameters like linearity, range, limit of detection, limit of quantification, and recovery. [, , , , , , , , , ]
体外研究产品的免责声明和信息
请注意,BenchChem 上展示的所有文章和产品信息仅供信息参考。 BenchChem 上可购买的产品专为体外研究设计,这些研究在生物体外进行。体外研究,源自拉丁语 "in glass",涉及在受控实验室环境中使用细胞或组织进行的实验。重要的是要注意,这些产品没有被归类为药物或药品,他们没有得到 FDA 的批准,用于预防、治疗或治愈任何医疗状况、疾病或疾病。我们必须强调,将这些产品以任何形式引入人类或动物的身体都是法律严格禁止的。遵守这些指南对确保研究和实验的法律和道德标准的符合性至关重要。


![5-[3-(dimethylamino)propyl]-5H-dibenzo[a,d]cyclohepten-5-ol](/img/structure/B195662.png)