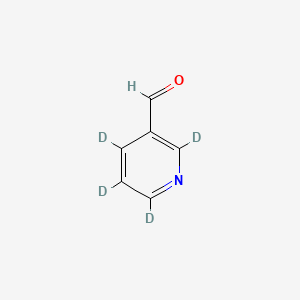
Gefitinib-d3
概述
描述
Gefitinib-d3 is a deuterated form of gefitinib, a tyrosine kinase inhibitor used primarily in the treatment of non-small cell lung cancer (NSCLC). The deuterium atoms in this compound replace hydrogen atoms, which can enhance the compound’s metabolic stability and pharmacokinetic properties. Gefitinib itself targets the epidermal growth factor receptor (EGFR), which is often overexpressed in certain types of cancer cells .
作用机制
Target of Action
Gefitinib-d3 primarily targets the Epidermal Growth Factor Receptor (EGFR) . EGFR is a protein that is often overexpressed in certain human carcinoma cells, such as lung and breast cancer cells . It plays a crucial role in cell growth and proliferation .
Mode of Action
This compound is a type of targeted cancer drug known as a tyrosine kinase inhibitor (TKI) . It works by inhibiting the intracellular phosphorylation of numerous tyrosine kinases associated with transmembrane cell surface receptors, including the tyrosine kinases associated with EGFR . This compound binds to the adenosine triphosphate (ATP)-binding site of the enzyme, thereby blocking signal transduction and resulting in the inhibition of cell proliferation .
Biochemical Pathways
The primary biochemical pathway affected by this compound is the EGFR signaling pathway . By inhibiting EGFR, this compound blocks the signals that tell cancer cells to grow . This leads to the inhibition of cell proliferation and the induction of apoptosis in cancer cells .
Pharmacokinetics
This compound is extensively metabolized in the body . It reaches the maximum plasma level relatively fast and is distributed extensively . The compound undergoes extensive biotransformation and is predominantly excreted in feces, with less than 7% excreted in the urine . The major enzyme involved in the metabolism of this compound is CYP3A4 .
Result of Action
The molecular effect of this compound is the inhibition of the EGFR tyrosine kinase, which leads to the blocking of signal transduction . This results in the inhibition of cell proliferation and the induction of apoptosis in cancer cells . On a cellular level, this compound selectively targets the mutant proteins in malignant cells .
Action Environment
The action, efficacy, and stability of this compound can be influenced by various environmental factors. The use of this compound is predicted to present an insignificant risk to the environment . The environmental risk is based on the Predicted Environmental Concentration (PEC) and the Predicted No Effect Concentration (PNEC) ratio
生化分析
Biochemical Properties
Gefitinib-d3 interacts with various enzymes and proteins, primarily the EGFR tyrosine kinase. It binds to the adenosine triphosphate (ATP)-binding site of the enzyme, thereby inhibiting the intracellular phosphorylation of numerous tyrosine kinases associated with transmembrane cell surface receptors . This interaction disrupts the signaling pathways that promote cell growth and proliferation, leading to the inhibition of cancer cell growth .
Cellular Effects
This compound has significant effects on various types of cells and cellular processes. It influences cell function by impacting cell signaling pathways, gene expression, and cellular metabolism . For instance, it inhibits the EGFR signaling pathway, leading to the suppression of downstream pathways such as MAPK and AKT, which are crucial for cell proliferation and survival .
Molecular Mechanism
The molecular mechanism of action of this compound involves its binding interactions with biomolecules, enzyme inhibition or activation, and changes in gene expression . By binding to the ATP-binding site of EGFR, it inhibits the activation of the receptor, thereby preventing the initiation of downstream signaling pathways that promote cell growth and proliferation .
Temporal Effects in Laboratory Settings
Over time, this compound demonstrates stability and long-term effects on cellular function in both in vitro and in vivo studies
Dosage Effects in Animal Models
In animal models, the effects of this compound vary with different dosages . Lower doses can effectively inhibit tumor growth, while higher doses may lead to toxic or adverse effects
Metabolic Pathways
This compound is involved in various metabolic pathways. It interacts with enzymes such as CYP3A5 and CYP2D6, which play a role in its metabolism . It also affects metabolic flux and metabolite levels, as seen in its impact on lipid metabolism and cholesterol biosynthesis .
Transport and Distribution
This compound is transported and distributed within cells and tissues through various mechanisms . It interacts with transporters such as the ATP-binding cassette and the solute carrier superfamily . These interactions can affect the localization or accumulation of this compound within cells.
Subcellular Localization
It is known that distinct N-terminal fusion partners drive differential subcellular localization, which imparts distinct cell signaling and oncogenic properties of different, clinically relevant ROS1 RTK fusion oncoproteins .
准备方法
Synthetic Routes and Reaction Conditions
The synthesis of gefitinib-d3 involves the incorporation of deuterium atoms into the gefitinib molecule. This can be achieved through various methods, including:
Hydrogen-Deuterium Exchange: This method involves the replacement of hydrogen atoms with deuterium in the presence of a deuterating agent.
Deuterated Reagents: Using deuterated reagents in the synthesis process can directly introduce deuterium atoms into the molecule.
Industrial Production Methods
Industrial production of this compound typically involves large-scale synthesis using deuterated reagents and optimized reaction conditions to ensure high yield and purity. The process may include steps such as:
Deuterium Gas Exchange: Utilizing deuterium gas in the presence of a catalyst to replace hydrogen atoms.
Deuterated Solvents: Employing deuterated solvents in the reaction mixture to facilitate the incorporation of deuterium.
化学反应分析
Types of Reactions
Gefitinib-d3 can undergo various chemical reactions, including:
Oxidation: The compound can be oxidized to form metabolites.
Reduction: Reduction reactions can modify the functional groups within the molecule.
Substitution: Substitution reactions can occur at specific sites on the molecule, leading to the formation of derivatives.
Common Reagents and Conditions
Oxidizing Agents: Such as hydrogen peroxide or potassium permanganate.
Reducing Agents: Such as sodium borohydride or lithium aluminum hydride.
Substitution Reagents: Such as halogens or alkylating agents.
Major Products
The major products formed from these reactions include various metabolites and derivatives of this compound, which can be analyzed for their pharmacological properties.
科学研究应用
Gefitinib-d3 has a wide range of scientific research applications, including:
Chemistry: Used as a reference standard in analytical chemistry to study the metabolic pathways and stability of gefitinib.
Biology: Employed in cellular and molecular biology research to investigate the effects of EGFR inhibition on cell signaling pathways.
Medicine: Utilized in pharmacokinetic and pharmacodynamic studies to understand the drug’s behavior in the body.
Industry: Applied in the development of new therapeutic agents and in the optimization of drug formulations.
相似化合物的比较
Similar Compounds
Erlotinib: Another EGFR tyrosine kinase inhibitor used in the treatment of NSCLC.
Afatinib: An irreversible EGFR inhibitor that targets multiple members of the ErbB family.
Osimertinib: A third-generation EGFR inhibitor effective against T790M resistance mutations.
Uniqueness
Gefitinib-d3 is unique due to the incorporation of deuterium atoms, which can enhance its metabolic stability and reduce the rate of metabolic degradation. This can potentially lead to improved pharmacokinetic properties and therapeutic efficacy compared to its non-deuterated counterpart .
属性
CAS 编号 |
1173976-40-3 |
|---|---|
分子式 |
C22H24ClFN4O3 |
分子量 |
449.926 |
IUPAC 名称 |
N-(3-chloro-4-fluorophenyl)-6-(3-morpholin-4-ylpropoxy)-7-(trideuteriomethoxy)quinazolin-4-amine |
InChI |
InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27)/i1D3 |
InChI 键 |
XGALLCVXEZPNRQ-FIBGUPNXSA-N |
SMILES |
COC1=C(C=C2C(=C1)N=CN=C2NC3=CC(=C(C=C3)F)Cl)OCCCN4CCOCC4 |
同义词 |
N-(3-Chloro-4-fluorophenyl)-7-(methoxy-d3)-6-[3-(4-morpholinyl)propoxy]-4-quinazolinamine; Iressa-d3; ZD 1839-d3; |
产品来源 |
United States |
Synthesis routes and methods I
Procedure details







Synthesis routes and methods II
Procedure details





体外研究产品的免责声明和信息
请注意,BenchChem 上展示的所有文章和产品信息仅供信息参考。 BenchChem 上可购买的产品专为体外研究设计,这些研究在生物体外进行。体外研究,源自拉丁语 "in glass",涉及在受控实验室环境中使用细胞或组织进行的实验。重要的是要注意,这些产品没有被归类为药物或药品,他们没有得到 FDA 的批准,用于预防、治疗或治愈任何医疗状况、疾病或疾病。我们必须强调,将这些产品以任何形式引入人类或动物的身体都是法律严格禁止的。遵守这些指南对确保研究和实验的法律和道德标准的符合性至关重要。
![6-Benzyl-1,2,3,4-tetrahydro-6H-pyrrolo[3,4-B]pyridine-5,7-dione](/img/structure/B563405.png)
![6-Benzyl-1-tert-boc-octahydropyrrolo[3,4-b]pyridine-d4](/img/structure/B563406.png)