Trimethoprim-d9 (Major)
Overview
Description
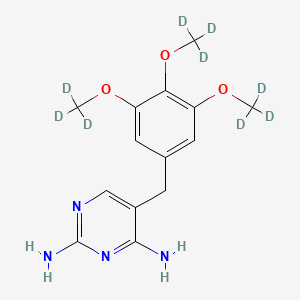
Trimethoprim-d9 (Major), also known as Trimethoprim-d9 (Major), is a useful research compound. Its molecular formula is C14H18N4O3 and its molecular weight is 299.378. The purity is usually 95%.
BenchChem offers high-quality Trimethoprim-d9 (Major) suitable for many research applications. Different packaging options are available to accommodate customers' requirements. Please inquire for more information about Trimethoprim-d9 (Major) including the price, delivery time, and more detailed information at [email protected].
Mechanism of Action
Target of Action
Trimethoprim-d9, also known as Trimethoprim-d9 (Major), primarily targets the bacterial enzyme dihydrofolate reductase (DHFR) . DHFR is a critical enzyme that catalyzes the formation of tetrahydrofolic acid (THF), an essential precursor in the biosynthesis of nucleic acids .
Mode of Action
Trimethoprim-d9 inhibits DHFR, thereby preventing the synthesis of bacterial DNA and ultimately leading to bacterial death . It binds with a much stronger affinity to bacterial DHFR compared to its mammalian counterpart, allowing Trimethoprim-d9 to selectively interfere with bacterial biosynthetic processes .
Biochemical Pathways
The inhibition of DHFR by Trimethoprim-d9 disrupts the biosynthesis pathways of thymidylate and purines, as well as several other amino acids like glycine, methionine, serine, and N-formyl-methionyl tRNA . This leads to an imbalance in the pathways involved in active synthesizing thymidylate, disrupts DNA replication, and eventually causes cell death .
Pharmacokinetics
Trimethoprim-d9 is a potent inhibitor of multidrug and toxin extrusion protein (MATE) and a weak inhibitor of cytochrome P450 (CYP) 2C8 . These properties can influence the absorption, distribution, metabolism, and excretion (ADME) of the compound, impacting its bioavailability .
Result of Action
The molecular and cellular effects of Trimethoprim-d9’s action include the inhibition of bacterial DNA synthesis, leading to bacterial death . Some of the new analogs of Trimethoprim-d9 inhibited DHFR activity more strongly than Trimethoprim did, indicating that the addition of amide bonds into the analogs of Trimethoprim-d9 increases their affinity towards DHFR .
Action Environment
Environmental factors can influence the action, efficacy, and stability of Trimethoprim-d9. For instance, pH plays a role in the mode of action of Trimethoprim-d9 on Escherichia coli . Moreover, soil-related factors, animal husbandry and waste management, potable and wastewater, and food safety can contribute to antimicrobial resistance . These factors need to be considered in modeling the fate and transport of Trimethoprim-d9 in coastal/estuarine waters .
Biochemical Analysis
Biochemical Properties
Trimethoprim-d9 interacts with the enzyme dihydrofolate reductase (DHFR), which plays a crucial role in the biosynthesis pathways of thymidylate, purines, and several amino acids . The interaction between Trimethoprim-d9 and DHFR inhibits the enzyme’s activity, disrupting DNA replication and eventually leading to cell death .
Cellular Effects
The effects of Trimethoprim-d9 on cells are primarily due to its inhibition of DHFR. This disruption in folate metabolism leads to an imbalance in the pathways involved in synthesizing thymidylate, which is essential for DNA replication . As a result, the function of cells is significantly affected, leading to cell death .
Molecular Mechanism
Trimethoprim-d9 exerts its effects at the molecular level by binding to the DHFR enzyme. This binding inhibits the enzyme’s activity, preventing the reduction of dihydrofolate acid to tetrahydrofolic acid . This disruption in folate metabolism leads to an imbalance in the pathways involved in synthesizing thymidylate, which is essential for DNA replication .
Metabolic Pathways
Trimethoprim-d9 is involved in the folate metabolism pathway. It interacts with the DHFR enzyme, which catalyzes the reduction of dihydrofolate acid to tetrahydrofolic acid . The inhibition of DHFR by Trimethoprim-d9 disrupts this metabolic pathway, leading to an imbalance in the pathways involved in synthesizing thymidylate .
Properties
IUPAC Name |
5-[[3,4,5-tris(trideuteriomethoxy)phenyl]methyl]pyrimidine-2,4-diamine | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C14H18N4O3/c1-19-10-5-8(6-11(20-2)12(10)21-3)4-9-7-17-14(16)18-13(9)15/h5-7H,4H2,1-3H3,(H4,15,16,17,18)/i1D3,2D3,3D3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
IEDVJHCEMCRBQM-GQALSZNTSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
COC1=CC(=CC(=C1OC)OC)CC2=CN=C(N=C2N)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
[2H]C([2H])([2H])OC1=CC(=CC(=C1OC([2H])([2H])[2H])OC([2H])([2H])[2H])CC2=CN=C(N=C2N)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C14H18N4O3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID10662219 | |
| Record name | Trimethoprim-d9 | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID10662219 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
299.37 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
1189460-62-5 | |
| Record name | Trimethoprim-d9 | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID10662219 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Synthesis routes and methods I
Procedure details








Synthesis routes and methods II
Procedure details





Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.




![[(Z)-[(E)-4-[(1R)-2,6,6-trimethylcyclohex-2-en-1-yl]but-3-en-2-ylidene]amino]urea](/img/structure/B562148.png)



![(1R,2R,4aS,6aR,6aS,6bR,8aR,9S,10R,11R,12aR,14bS)-9-[[2-[(2S,3R,4S)-3-ethenyl-5-methoxycarbonyl-2-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-3,4-dihydro-2H-pyran-4-yl]acetyl]oxymethyl]-1,10,11-trihydroxy-1,2,6a,6b,9,12a-hexamethyl-2,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylic acid](/img/structure/B562155.png)




