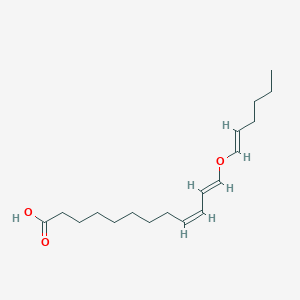
Simeprevir
Overview
Description
TMC435, also known as simeprevir, is a potent, once-daily, noncovalent, oral hepatitis C virus NS3/4A protease inhibitor. It is used in combination with pegylated interferon and ribavirin for the treatment of chronic hepatitis C virus genotype 1 infection. This compound has been approved for use in several countries, including Japan, Canada, the United States, Russia, the European Union, Mexico, and Australia .
Mechanism of Action
Target of Action
Simeprevir is a direct-acting antiviral agent that primarily targets the Hepatitis C Virus (HCV) NS3/4A protease . This protease is essential for the viral replication process of HCV, particularly for genotypes 1 and 4 .
Mode of Action
This compound inhibits the HCV NS3/4A protease in a potent and highly specific manner . This protease is responsible for cleaving the HCV-encoded polyprotein into individual viral proteins, which is a critical step in the HCV viral life cycle . By inhibiting this protease, this compound effectively blocks the viral replication process .
Biochemical Pathways
The inhibition of the HCV NS3/4A protease disrupts the viral replication process, preventing the maturation of the virus . This compound also shows synergistic effects with interferon-α and HCV NS5B inhibitor, and additive effects with ribavirin in HCV replicon cells .
Pharmacokinetics
This compound is orally bioavailable, and its absorption increases when taken with food . It is primarily metabolized by the liver’s CYP3A4 enzymes, but CYP2C8 and CYP2C19 enzymes can also play a role . The half-life of this compound in the plasma is 41 hours in people with HCV . It is excreted mainly through feces (91%) and less than 1% through urine .
Result of Action
The inhibition of the HCV NS3/4A protease by this compound results in the disruption of the viral replication process, thereby preventing the maturation of the virus . This leads to a decrease in the viral load and can result in a sustained virologic response (SVR), which is considered a cure for HCV .
Action Environment
The efficacy of this compound can be influenced by certain polymorphic variants of the virus. Furthermore, the environment within the host’s body, such as the presence of other medications or liver function, can also impact the action and efficacy of this compound .
Biochemical Analysis
Biochemical Properties
Simeprevir plays a crucial role in inhibiting the replication of the hepatitis C virus by targeting the NS3/4A protease, an enzyme essential for the viral life cycle. The compound binds non-covalently to the NS3/4A protease, resulting in a fast association and slow dissociation rate . This interaction prevents the protease from cleaving the HCV polyprotein into functional viral proteins, thereby inhibiting viral replication. This compound also interacts with other biomolecules, such as the cofactor N4A subunit, which is part of the NS3/4A heterodimeric complex .
Cellular Effects
This compound exerts significant effects on various types of cells, particularly hepatocytes, where it accumulates after uptake via organic anion-transporting polypeptides OATP1B1 and OATP1B3 . In hepatocytes, this compound inhibits the NS3/4A protease, leading to a reduction in viral replication. This inhibition affects cell signaling pathways, gene expression, and cellular metabolism by preventing the production of viral proteins necessary for the virus’s life cycle . Additionally, this compound has been shown to display synergistic effects with interferon-α and HCV NS5B inhibitors, further enhancing its antiviral activity .
Molecular Mechanism
The molecular mechanism of this compound involves its binding to the NS3/4A protease, a critical enzyme for the hepatitis C virus. By inhibiting this protease, this compound prevents the cleavage of the HCV polyprotein into individual viral proteins, thereby blocking viral replication . This compound’s binding interactions with the NS3/4A protease are highly specific and potent, making it an effective antiviral agent. Additionally, this compound’s resistance profile differs from first-generation protease inhibitors, as it is less effective against certain polymorphic variants of the NS3 protease .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of this compound have been observed to change over time. The compound is relatively stable, with a half-life of approximately 41 hours in individuals with HCV . Over time, this compound’s antiviral activity can be influenced by factors such as drug degradation and the development of viral resistance. Long-term studies have shown that this compound maintains its efficacy in reducing viral load and achieving sustained virological response (SVR) when used in combination with other antiviral agents .
Dosage Effects in Animal Models
The effects of this compound vary with different dosages in animal models. Studies have shown that higher doses of this compound result in more significant reductions in viral replication and improved treatment outcomes . At very high doses, this compound may exhibit toxic or adverse effects, such as hepatotoxicity. It is essential to determine the optimal dosage that maximizes antiviral efficacy while minimizing potential side effects .
Metabolic Pathways
This compound is primarily metabolized in the liver by the cytochrome P450 enzyme CYP3A4, with minor contributions from CYP2C8 and CYP2C19 . The metabolic pathways of this compound involve its conversion into various metabolites, which are then excreted from the body. The compound’s interaction with these enzymes can affect metabolic flux and metabolite levels, influencing its overall efficacy and safety profile .
Transport and Distribution
This compound is transported and distributed within cells and tissues through specific transporters and binding proteins. The compound is taken up into hepatocytes via organic anion-transporting polypeptides OATP1B1 and OATP1B3, where it accumulates and exerts its antiviral effects . This compound’s localization within hepatocytes is crucial for its activity, as it needs to reach the NS3/4A protease to inhibit viral replication effectively .
Subcellular Localization
The subcellular localization of this compound is primarily within the cytoplasm of hepatocytes, where it interacts with the NS3/4A protease . The compound’s activity and function are influenced by its localization, as it needs to be in proximity to the viral protease to exert its inhibitory effects. Post-translational modifications and targeting signals may also play a role in directing this compound to specific compartments within the cell .
Preparation Methods
The preparation of TMC435 involves several synthetic routes and reaction conditions. One of the key intermediates in the synthesis is a bicyclic lactone amide. The preparation method for this intermediate involves multiple steps, including the formation of a macrocyclic compound . The industrial production of TMC435 typically involves the crystallization of the compound to obtain a stable and pure form suitable for pharmaceutical use .
Chemical Reactions Analysis
TMC435 undergoes various chemical reactions, including oxidation, reduction, and substitution. Common reagents used in these reactions include organic solvents, acids, and bases. The major products formed from these reactions are typically derivatives of the original compound, which may have different pharmacological properties .
Scientific Research Applications
TMC435 has a wide range of scientific research applications. It is primarily used in the treatment of chronic hepatitis C virus infection. The compound has been extensively studied in clinical trials to evaluate its efficacy, safety, and pharmacokinetics. Additionally, TMC435 is used in research to understand the resistance profile of hepatitis C virus and to develop new antiviral therapies .
Comparison with Similar Compounds
TMC435 is similar to other hepatitis C virus NS3/4A protease inhibitors, such as telaprevir and boceprevir. TMC435 has several unique features that distinguish it from these compounds. For example, TMC435 has a more favorable resistance profile and is effective against a broader range of hepatitis C virus genotypes. Additionally, TMC435 has been shown to have a better safety and tolerability profile compared to other protease inhibitors .
Similar Compounds
- Telaprevir
- Boceprevir
- Grazoprevir
- Paritaprevir
Properties
CAS No. |
923604-59-5 |
|---|---|
Molecular Formula |
C38H47N5O7S2 |
Molecular Weight |
749.9 g/mol |
IUPAC Name |
(4S)-N-cyclopropylsulfonyl-17-[7-methoxy-8-methyl-2-(4-propan-2-yl-1,3-thiazol-2-yl)quinolin-4-yl]oxy-13-methyl-2,14-dioxo-3,13-diazatricyclo[13.3.0.04,6]octadec-7-ene-4-carboxamide |
InChI |
InChI=1S/C38H47N5O7S2/c1-21(2)30-20-51-35(40-30)29-18-32(26-13-14-31(49-5)22(3)33(26)39-29)50-24-16-27-28(17-24)36(45)43(4)15-9-7-6-8-10-23-19-38(23,41-34(27)44)37(46)42-52(47,48)25-11-12-25/h8,10,13-14,18,20-21,23-25,27-28H,6-7,9,11-12,15-17,19H2,1-5H3,(H,41,44)(H,42,46)/t23?,24?,27?,28?,38-/m0/s1 |
InChI Key |
JTZZSQYMACOLNN-ZMKZURCUSA-N |
SMILES |
CC1=C(C=CC2=C1N=C(C=C2OC3CC4C(C3)C(=O)N(CCCCC=CC5CC5(NC4=O)C(=O)NS(=O)(=O)C6CC6)C)C7=NC(=CS7)C(C)C)OC |
Isomeric SMILES |
CC1=C(C=CC2=C1N=C(C=C2OC3CC4C(C3)C(=O)N(CCCCC=CC5C[C@@]5(NC4=O)C(=O)NS(=O)(=O)C6CC6)C)C7=NC(=CS7)C(C)C)OC |
Canonical SMILES |
CC1=C(C=CC2=C1N=C(C=C2OC3CC4C(C3)C(=O)N(CCCCC=CC5CC5(NC4=O)C(=O)NS(=O)(=O)C6CC6)C)C7=NC(=CS7)C(C)C)OC |
Color/Form |
White to almost white powder |
Pictograms |
Irritant; Environmental Hazard |
solubility |
Insoluble Practically insoluble in water over a wide pH range Practically insoluble in propylene glycol; very slightly soluble in ethanol; slightly soluble in acetone. Soluble in dichloromethane; freely soluble in some organic solvents |
Synonyms |
435, TMC 435350, TMC N-(17-(2-(4-isopropylthiazole-2-yl)-7-methoxy-8-methylquinolin-4-yloxy)-13-methyl-2,14-dioxo-3,13-diazatricyclo(13.3.0.04,6)octadec-7-ene-4-carbonyl)(cyclopropyl)sulfonamide Olysio simeprevir TMC 435 TMC 435350 TMC-435 TMC-435350 TMC435 TMC435350 |
vapor_pressure |
5.9X10-27 mm Hg at 25 °C (est) |
Origin of Product |
United States |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.






![Calix[5]pyrrole](/img/structure/B1263095.png)


![[(2R)-2,3-di(hexadecanoyloxy)propyl] (9Z,12Z)-octadeca-9,12-dienoate](/img/structure/B1263103.png)