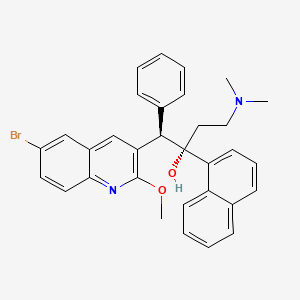
Bedaquiline
Overview
Description
Bedaquiline is a diarylquinoline antimycobacterial drug used primarily for the treatment of multidrug-resistant tuberculosis. It was approved for medical use in the United States in 2012 and is listed on the World Health Organization’s List of Essential Medicines . This compound works by inhibiting the mycobacterial ATP synthase enzyme, which is essential for the energy production of Mycobacterium tuberculosis .
Mechanism of Action
Target of Action
Bedaquiline, a diarylquinoline antimycobacterial drug, primarily targets the c subunit of ATP synthase . This enzyme is responsible for synthesizing ATP, a crucial energy molecule, in Mycobacterium tuberculosis .
Mode of Action
This compound inhibits the c subunit of ATP synthase, thereby disrupting the synthesis of ATP . This inhibition leads to an energy deficit within the mycobacterial cells, which can result in cell death .
Biochemical Pathways
The primary biochemical pathway affected by this compound is the ATP synthesis pathway . By inhibiting ATP synthase, this compound disrupts the energy production within the mycobacterial cells . This disruption can lead to a variety of downstream effects, including impaired cellular functions and eventual cell death .
Pharmacokinetics
This compound is metabolized primarily by the cytochrome P450 isoenzyme 3A4 (CYP3A4) to a less-active N-monodesmethyl metabolite . It displays linear pharmacokinetics following single-dose administration up to a dose of at least 700 mg and multiple-dose administration up to a dose of at least 400 mg once daily . After 8 weeks of this compound administration in patients with MDR-TB, the mean terminal half-life was 164 days for this compound and 159 days for its metabolite . These pharmacokinetic properties influence the bioavailability of this compound and its therapeutic effects .
Result of Action
The inhibition of ATP synthase by this compound results in a significant energy deficit within the mycobacterial cells . This energy deficit impairs various cellular functions, leading to the death of the mycobacterial cells . As a result, this compound exhibits high in vitro activity against M.
Action Environment
This compound’s action can be influenced by various environmental factors. For instance, it is greatly influenced by cytochrome P450 inducers and inhibitors, which can affect its metabolism and, consequently, its efficacy . Additionally, patient demographics and comorbidities may influence the observed concentrations of this compound . Therefore, understanding these factors is crucial for optimizing the use of this compound in treating tuberculosis .
Biochemical Analysis
Biochemical Properties
Bedaquiline interacts with the ATP synthase enzyme of the Mycobacterium tuberculosis, specifically inhibiting the c subunit responsible for synthesizing ATP . It is metabolized primarily by the cytochrome P450 isoenzyme 3A4 (CYP3A4) to a less-active N-monodesmethyl metabolite . In addition to CYP3A4, both CYP2C8 and CYP2C19 contribute to this compound N-demethylation .
Cellular Effects
This compound has a bactericidal effect on Mycobacterium tuberculosis. It interferes with the function of an enzyme required by the tuberculosis bacterium to produce energy and replicate . It has been shown to increase the number of lysosomes within macrophage cells and the activity of genes and proteins that increase lysosomes’ ability to break down foreign particles .
Molecular Mechanism
This compound blocks the proton pump for ATP synthase of mycobacteria . It binds to the transmembrane F0 region of ATP synthase, blocking the proton flow through the enzyme and the conformational changes of the enzyme . As a result, the ATP production at the hexamer is inhibited .
Temporal Effects in Laboratory Settings
This compound concentrations remain fairly stable over the treatment period, especially when considering the dosage alteration after 2 weeks of treatment initiation . In vitro experiments have indicated that this compound may also target the mitochondrial ATP synthase of malignant mammalian cells and reduce the rate of metastasis .
Dosage Effects in Animal Models
Studies in the murine model of TB infection evaluating oral administration of different dosages and different dosing frequencies indicated that the bactericidal activity of this compound is concentration-dependent and that the AUC is the main pharmacokinetic/pharmacodynamic driver for efficacy .
Metabolic Pathways
This compound is hepatically metabolized, primarily by cytochrome 450 (CYP) 3A4 with additional involvement from CYP2C8 and CYP2C19 . M2 is an active metabolite but demonstrates activity to approximately fivefold less than the parent compound .
Transport and Distribution
In human plasma samples, this compound is >99.9% bound to protein at concentrations of ≥5 mg/L . This compound also binds tightly to plasma protein in other species (mice, rats, dogs, monkeys, and rabbits) .
Subcellular Localization
It is known that this compound interferes with the function of an enzyme required by the tuberculosis bacterium to produce energy and replicate .
Preparation Methods
Synthetic Routes and Reaction Conditions: The synthesis of bedaquiline involves the reaction of 3-benzyl-6-bromo-2-methoxyquinoline with 3-(dimethylamino)-1-(naphthalen-1-yl)propan-1-one in the presence of lithium pyrrolidide . Another method involves reacting a compound II and a compound III in tetrahydrofuran with zinc powder under the action of a catalyst such as trimethylchlorosilane or 1,2-dibromoethane .
Industrial Production Methods: Industrial production of this compound often employs spray drying techniques to prepare respirable particles for pulmonary delivery. This method involves the use of excipients like L-leucine to enhance the fine particle fraction suitable for inhalation therapies .
Chemical Reactions Analysis
Types of Reactions: Bedaquiline undergoes oxidative metabolism primarily by the cytochrome P450 isoenzyme 3A4, leading to the formation of a less-active N-monodesmethyl metabolite . It can also undergo protonation and dissociation in aqueous solutions, forming various soluble species depending on the pH .
Common Reagents and Conditions:
Oxidation: Cytochrome P450 isoenzyme 3A4
Protonation and Dissociation: Pure water, pH range 2-7
Major Products:
N-monodesmethyl metabolite (M2): Formed through oxidative metabolism
Soluble species (L-, LH, LH2+, LH32+, LH43+): Formed through protonation and dissociation
Scientific Research Applications
Bedaquiline is primarily used in the treatment of multidrug-resistant tuberculosis. It has shown efficacy in achieving higher culture conversion rates and reducing mortality among drug-resistant tuberculosis cases . Additionally, this compound is being studied for its potential to improve first-line treatment for rifampicin-susceptible tuberculosis by reducing treatment duration . Its unique mechanism of action also makes it a candidate for modification to target other clinically important bacteria .
Comparison with Similar Compounds
- Isoniazid
- Rifampicin
- Pyrazinamide
- Ethambutol
- Kanamycin
- Ofloxacin
- Cycloserine
Comparison: Bedaquiline is unique among these compounds due to its mechanism of action targeting ATP synthase, whereas other drugs like isoniazid and rifampicin target different pathways such as mycolic acid synthesis and RNA synthesis, respectively . This compound’s ability to inhibit both actively replicating and dormant mycobacterial cells further distinguishes it from other antimycobacterial agents .
Properties
IUPAC Name |
(1R,2S)-1-(6-bromo-2-methoxyquinolin-3-yl)-4-(dimethylamino)-2-naphthalen-1-yl-1-phenylbutan-2-ol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C32H31BrN2O2/c1-35(2)19-18-32(36,28-15-9-13-22-10-7-8-14-26(22)28)30(23-11-5-4-6-12-23)27-21-24-20-25(33)16-17-29(24)34-31(27)37-3/h4-17,20-21,30,36H,18-19H2,1-3H3/t30-,32-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
QUIJNHUBAXPXFS-XLJNKUFUSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CN(C)CCC(C1=CC=CC2=CC=CC=C21)(C(C3=CC=CC=C3)C4=C(N=C5C=CC(=CC5=C4)Br)OC)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CN(C)CC[C@@](C1=CC=CC2=CC=CC=C21)([C@H](C3=CC=CC=C3)C4=C(N=C5C=CC(=CC5=C4)Br)OC)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C32H31BrN2O2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID101027810, DTXSID80903989 | |
| Record name | rel-(1R,2S)-1-(6-Bromo-2-methoxyquinolin-3-yl)-4-(dimethylamino)-2-(naphthalen-1-yl)-1-phenylbutan-2-ol | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID101027810 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Bedaquiline | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID80903989 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
555.5 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
Insoluble | |
| Record name | Bedaquiline | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08903 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Mechanism of Action |
Bedaquiline is a diarylquinoline antimycobacterial drug that inhibits the proton pump of mycobacterial ATP (adenosine 5'-triphosphate) synthase, an enzyme that is essential for the generation of energy in Mycobacterium tuberculosis. Bacterial death occurs as a result of bedaquiline., Bedaquiline (BDQ), an ATP synthase inhibitor, is the first drug to be approved for treatment of multidrug-resistant tuberculosis in decades. Though BDQ has shown excellent efficacy in clinical trials, its early bactericidal activity during the first week of chemotherapy is minimal. Here, using microfluidic devices and time-lapse microscopy of Mycobacterium tuberculosis, we confirm the absence of significant bacteriolytic activity during the first 3-4 days of exposure to BDQ. BDQ-induced inhibition of ATP synthesis leads to bacteriostasis within hours after drug addition. Transcriptional and proteomic analyses reveal that M. tuberculosis responds to BDQ by induction of the dormancy regulon and activation of ATP-generating pathways, thereby maintaining bacterial viability during initial drug exposure. BDQ-induced bacterial killing is significantly enhanced when the mycobacteria are grown on non-fermentable energy sources such as lipids (impeding ATP synthesis via glycolysis). Our results show that BDQ exposure triggers a metabolic remodelling in mycobacteria, thereby enabling transient bacterial survival., Bedaquiline is a diarylquinoline antimycobacterial drug that inhibits mycobacterial ATP (adenosine 5'-triphosphate) synthase, an enzyme that is essential for the generation of energy in Mycobacterium tuberculosis., Infections with Mycobacterium tuberculosis are substantially increasing on a worldwide scale and new antibiotics are urgently needed to combat concomitantly emerging drug-resistant mycobacterial strains. The diarylquinoline TMC207 /bedaquiline/ is a highly promising drug candidate for treatment of tuberculosis. This compound kills M. tuberculosis by binding to a new target, mycobacterial ATP synthase. In this study we used biochemical assays and binding studies to characterize the interaction between TMC207 and ATP synthase. We show that TMC207 acts independent of the proton motive force and does not compete with protons for a common binding site. The drug is active on mycobacterial ATP synthesis at neutral and acidic pH with no significant change in affinity between pH 5.25 and pH 7.5, indicating that the protonated form of TMC207 is the active drug entity. The interaction of TMC207 with ATP synthase can be explained by a one-site binding mechanism, the drug molecule thus binds to a defined binding site on ATP synthase. TMC207 affinity for its target decreases with increasing ionic strength, suggesting that electrostatic forces play a significant role in drug binding. Our results are consistent with previous docking studies and provide experimental support for a predicted function of TMC207 in mimicking key residues in the proton transfer chain and blocking rotary movement of subunit c during catalysis. Furthermore, the high affinity of TMC207 at low proton motive force and low pH values may in part explain the exceptional ability of this compound to efficiently kill mycobacteria in different microenvironments. | |
| Record name | Bedaquiline | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08903 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Bedaquiline | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8217 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
White solid | |
CAS No. |
843663-66-1, 654653-93-7 | |
| Record name | Bedaquiline | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=843663-66-1 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | rel-(αR,βS)-6-Bromo-α-[2-(dimethylamino)ethyl]-2-methoxy-α-1-naphthalenyl-β-phenyl-3-quinolineethanol | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=654653-93-7 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | 3-Quinolineethanol, 6-bromo-alpha-(2-(dimethylamino)ethyl)-2-methoxy-alpha-1-naphthalenyl-beta-phenyl-, (alphaR,betaS)-rel- | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0654653937 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Bedaquiline [USAN:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0843663661 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Bedaquiline | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08903 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | rel-(1R,2S)-1-(6-Bromo-2-methoxyquinolin-3-yl)-4-(dimethylamino)-2-(naphthalen-1-yl)-1-phenylbutan-2-ol | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID101027810 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Bedaquiline | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID80903989 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | (1R,2S)-1-(6-bromo-2-methoxy-3-quinolinyl)-4- (dimethylamino)-2-(1-naphthalenyl)-1-phenyl-2-butanol | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | BEDAQUILINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/78846I289Y | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Bedaquiline | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8217 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Melting Point |
118 °C | |
| Record name | Bedaquiline | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8217 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


![4-{[(4S,5R,6E,8E,10Z,13Z)-1-carboxy-4-hydroxynonadeca-6,8,10,13-tetraen-5-yl]sulfanyl}benzoic acid](/img/structure/B1667826.png)
![(5E)-5-[(E)-3-[4-(Dimethylamino)phenyl]prop-2-enylidene]-1-phenyl-1,3-diazinane-2,4,6-trione](/img/structure/B1667835.png)
![(3R)-4-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2R)-6-amino-2-[[(2S,3E)-2-[[(2S)-2-amino-3-(4-hydroxyphenyl)propanoyl]amino]hexa-3,5-dienyl]amino]hexanoyl]amino]-3-(1H-indol-3-yl)propanoyl]amino]hexanoyl]amino]-4-oxo-4-sulfooxybutanoyl]amino]-3-phenylpropan](/img/structure/B1667838.png)